
Instrumentation recreates properties of the myopic eye to test lenses that prevent visual decline.

Instrumentation recreates properties of the myopic eye to test lenses that prevent visual decline.

According to the company, study results show sustained reductions in both IOP and glaucoma medication use in mild-moderate primary open angle glaucoma patients treated with a procedure enabled with the OMNI Surgical System technology.

In a recent update, the CDC has noted an additional death, bringing the death toll up to 4, with numerous counts of vision loss across the US.

Data demonstrate a reduction in the loss of retinal pigmented epithelial and photoreceptor cells.


The two companies have announced an exclusive agreement for Polifarma to commercialize AVT06, the proposed biosimilar to Eylea, in Turkey.

According to the company, the Phase 1 trial is a multicenter, open-label, dose-escalation safety clinical trial. Up to 24 subjects will receive a single periocular injection and will undergo monthly evaluation for up to 6 months to assess safety, tolerability, and efficacy measured by best corrected visual acuity.

According to the company, the DIAMOND trial in diabetic macular edema with topical OCS-01 met its stage 1 objective of validating the loading and maintenance dosing regimen designed to optimize OCS-01 efficacy potential with robust statistical significance.

Study: AO retinal imaging has the potential to become a sensitive marker for disease.
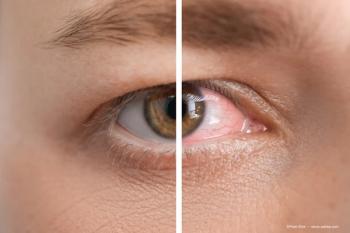
The company noted tanfanercept did not demonstrate statistical significance in either of the primary outcome measures of improvement in central corneal staining score or in improvement in Eye Dryness Score assessed at week 8 in subjects with dry eye disease, compared to vehicle. However, tanfanercept did demonstrate a highly statistically significant improvement on one of the secondary outcome measures, Schirmer testing of tear volume.
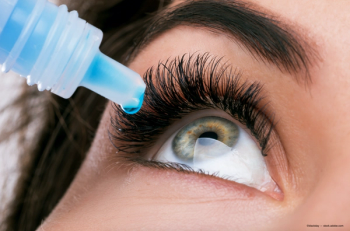
Ophthalmology Times reached out to members of its editorial advisory board along with ophthalmologists who specialize in dry eye for their comments on what this approval means for their patients with dry eye.

The number of cases continues to rise throughout the world

According to the companies, MIEBO is the first and only prescription eye drop approved for dry eye disease that directly targets tear evaporation, based on consistent results from a pair of pivotal Phase 3 trials.

During the 26th annual meeting of the American Society of Gene & Cell Therapy, taking place through Saturday in Los Angeles, California, the company is delivering several presentations highlighting its AAV capsids and gene constructs.

The lawsuit states the $27.8 billion acquisition would allow Amgen to strengthen their drug portfolio and stifle drug competition in a bid to monopolize two fast-growing medications.

The American Academy of Ophthalmology Truhlsen-Marmor Museum of the Eye, a free public museum dedicated to the science of sight, will present Decoding the Eye.: Signs and Symbols.

The company noted YUTIQ is used for the treatment of chronic non-infectious uveitis affecting the posterior segment of the eye, and is the same as Alimera’s ILUVIEN.
Clinical judgment is not always sufficient to determine whether a patient has an inherited corneal disorder.

In a presentation at the American Society of Cataract and Refractive Surgery meeting in San Diego, Kenneth A. Beckman, MD, FACS, discussed the current status of corneal crosslinking with some emerging treatments, including epi-on crosslinking.

Earlier this year, a federal civil jury concluded that the defendants violated the False Claims Act and the Anti-Kickback Statute by paying kickbacks to ophthalmic surgeons to induce their use of the defendants’ products in cataract surgeries reimbursed by Medicare.

Diana V. Do, MD, presented details of the PHOTON study at the Association for Research in Vision and Ophthalmology annual meeting, held recently in New Orleans, highlighting the safety and efficacy of 8 milligrams of aflibercept.

Groundbreaking treatment for patients with bullous keratopathy approved in Japan.

In November 2022, doctors in Cleveland diagnosed a patient with a corneal ulcer with a Pseudomona aeruginosa infection. The patient acquired the infection from tainted EzriCare eye drops months before the CDC’s February 2023 warning.

Debate continues over the best way to apply the treatment option.

According to the company, its new 20,000-square-foot facility in Hayward, California, includes cGMP suites, developmental and testing laboratories, equipped with single use bioreactors.

Jeff Wongs, MD, discussed his study to assess the benefits of cryopreserved amniotic membrane prior to cataract surgery to restore the ocular surface and improve the accuracy of pre-op biometric readings

Nonpowder guns, like NERF guns, are causing an increase in ocular injuries in children.

According to the U.S. Attorney’s Office, District of Rhode Island, Paul S. Koch, MD, former owner of several ophthalmology practices in the state, agreed to settle civil allegations he paid kickbacks to optometrists who referred patients to his practices for cataract surgeries.

The goal of the project is to encourage patients with Demodex blepharitis to visit an ophthalmologist.
