Dry Eye Awareness
Latest News

Study finds increased air pollution leads to surge in daily eye clinic visits
BostonSight publishes pilot study on PROSE scleral lenses as a drug delivery system for cyclosporine in dry eye treatment
Video Series

Latest Videos
CME Content
More News
Joanne F. Shen, MD, director of the dry eye clinic at Mayo Clinic's campus in Phoenix, Arizona, and a research team studied 35 patients treated with IPL/MGX. The team reviewed demographics, ocular histories, Standard Patient Evaluation of Eye Dryness 2 symptom survey scores, slit-lamp examinations and meibomian gland evaluations at baseline and at each visit before IPL/MGX treatments.

Most respondents were unsure of how to seek treatment and see dry eye as something that must be learned to live with.
According to researchers, insights into the ocular microbiome could have implications beyond eye health.

The company said the module, which includes real-world data on more than 10 million patients, will help advance research and accelerate therapy development for dry eye disease and other ocular surface disorders.

Walgreens Boots Allianc Inc. and CVS Health are among the companies the FDA sent letters to for violating federal law.
Researchers at Washington University School of Medicine in St. Louis have found that proteins made by stem cells that regenerate the cornea may be new targets for treating and preventing such injuries.

According to investigators, the risk for the development of dry eye disease is greater in postmenopausal women compared with those who have not reached menopause as a result of hormonal dysregulation of the ocular secretory glands.

According to the company, the OK-101 phase 2 trial will incorporate primary and secondary efficacy endpoints characterizing signs and symptoms of dry eye disease.

According to Jennifer Loh, MD, women achieve significantly better symptom relief than men.

Leading U.S. eye care experts advance eye health focused digital platform designed to make eye care part of daily self-care routine.

A potential first-in-class eye drop with a novel mechanism of action, NOV03 is an investigational therapy to treat the signs and symptoms of dry eye disease associated with Meibomian gland dysfunction.

Although predefined co-primary study endpoints were not met, AR-15512 demonstrated statistically significant improvements in DED signs, symptoms, and disease-related quality of life.
According to the company, topical eye drops anti-TNFα agent licaminlimab (OCS-02) relieve persistent ocular discomfort in severe dry eye disease.
An independent data monitoring committee recommends continuing study with a sample size target of up to 350 Patients. According to the company, no safety concerns have been identified and topline results are expected during the second quarter of 2023.

ThermaMEDx today announced a partnership with EyeCare Partners to expand EverTears distribution across the network of the national provider of clinically integrated eye care.

Tarsus Pharmaceuticals has enrolled the first patient in a Phase 2a clinical trial studying TP-03 for the treatment of meibomian gland disease in patients diagnosed with Demodex mites.

NOV03 is an investigational treatment with a proposed indication of treating the signs and symptoms of dry eye disease associated with Meibomian gland dysfunction.

The organization hopes to increase awareness and education of a condition that affects vision, mental health.
One obvious trend has been that dry eye disease is a concurrent problem for virtually all my patients, and we need to address it for optimum comfort and surgical results.
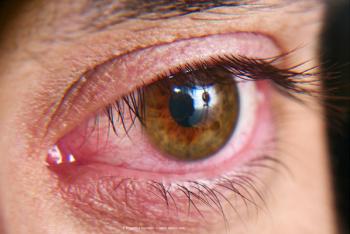
Top-line results are expected during second quarter for the therapeutic to treat patients with dry eye disease.
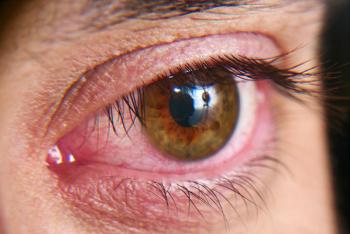

TearSolutions Inc. is preparing to conduct two trials, one in a primary Sjögren’s Syndrome patient population and the second in a potentially less severe general dry eye population for the treatment of DED.

Aldeyra Therapeutics announced today that it has achieved the primary endpoint of ocular redness in its randomly assigned, double-masked, vehicle-controlled Phase 2 clinical trial in dry eye disease.

A team of investigators at the Texas Tech University Health Sciences Center have developed a technology derived from corneal epithelial stem cells to improve outcomes for DED patients.

TearLab, MellingMedical reach an agreement to enhance early detection of dry eye disease in veterans.






























