
In the trial, PL9643 failed to meet co-primary and secondary endpoints.

Editor, Ophthalmology Times

In the trial, PL9643 failed to meet co-primary and secondary endpoints.

Steven R. Sarkisian, MD, sat down with David Hutton of Ophthalmology Times to discuss his poster about the Travoprost intraocular implant in phakic eyes from the American Glaucoma Society meeting held in Huntington Beach
I. Paul Singh, MD, sat down with David Hutton of Ophthalmology Times to discuss his poster "Travoprost Intraocular Implant Demonstrates Efficacy in Diverse Subpopulations of OAG and OHT Subjects," from the American Glaucoma Society meeting held in Huntington Beach

The supplement is specifically crafted to promote healthy intraocular pressure (IOP), nurturing the well-being of retinal ganglion cells, and sustain the health of the retinal nerve fiber layer while boosting memory and concentration.
Leon Herndon, MD, President of the American Glaucoma Society, sat down with David Hutton of Ophthalmology Times to discuss the AGS meeting held in Huntington Beach and what attendees can expect as well as new and exciting information to come from the meeting.

The patch is the world's first non-degradable, synthetic tissue substitute for ophthalmic surgery.
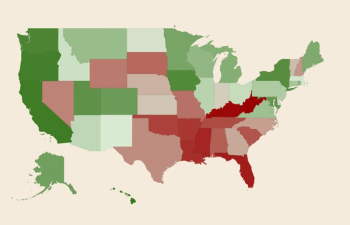
RX Safety conducted an analysis across all 50 US states and Washington DC to determine which state has the best eye health, as well as the worst.

The trial of esonadogene mvoparvovec (Opvika) will take place in the US for the treatment of Leber hereditary optic neuropathy caused by ND4 mutation (ND4-LHON).

Cutting-edge advances are outlined at Hawaiian Eye and Retina 2024

Products under the terms of the agreement include VERKAZIA, Cationorm PLUS, VEVYE, IHEEZO, and ZERVIATE.

David Hutton of Ophthalmology Times talks with Kathryn A. Colby, MD, PhD, Elisabeth J. Cohen Professor and Chair, NYU Langone Department of Ophthalmology, about the year the department had in 2023 as well as an outlook at advancements in the field of ophthalmology moving forward.

Chao Zhou, PhD, a professor of biomedical engineering in the McKelvey School of Engineering at the university, and team received the award from the Advanced Research Projects Agency for Health (ARPA-H).

David Hutton of Ophthalmology Times talks with Taylor Strange, MD, about his time building his practice and implementing the use of the LENSAR ALLY system.

David Hutton of Ophthalmology Times talks with Brian Strem, PhD, CEO Kiora Pharmaceuticals, about their recent partnership with Théa Open Innovation and further development of their KIO-301 and KOI-104 programs.
Joseph Panarelli, MD, sat down with David Hutton of Ophthalmology Times to discuss the panel he moderated at the Glaucoma 360 meeting held in San Francisco, about innovations in glaucoma drugs, and drug delivery.

The trial will evaluate tivozanib eye drops (KHK4951), a small-molecule vascular endothelial growth factor receptor (VEGFR)-1, -2, and -3 tyrosine kinase inhibitor (TKI), in patients in with DME.

A third party report regarding the school’s ophthalmology program showed an environment of “anxiety, apprehension, disappointment, frustration, fear, regret.”

Anat Loewenstein, MD, sat down with Hattie Hayes of Ophthalmology Times Europe to discuss the implication of longitudinal AI-based fluid quantification for at-home monitoring from the Angiogenesis Exudation and Degeneration 2024 event.
Carl Danzig, MD, sat down with Martin David Harp of Ophthalmology Times to discuss his presentation AVD-104 for Geographic Atrophy: Glyco-immunologic modulation of macrophage activity from the Angiogenesis Exudation and Degeneration 2024 event.

SriniVas R. Sadda, MD, sat down with David Hutton of Ophthalmology Times to discuss his presentation about precursor lesions for development of atrophy and AMD from the Angiogenesis Exudation and Degeneration 2024 event.

David Hutton of Ophthalmology Times talks with Jeffrey Cleland, PhD, President and CEO of Ashvattha Therapeutics, about the company's D-4517.2, a unique nanomedicine technology for the treatment of wet AMD and DME.

The recall is “due to potential safety concerns after FDA investigators found insanitary conditions.”

The app is a tool aimed at allowing patients to measure their visual acuity at home by themselves.
Kamuvudines are a new class of inflammasome inhibitor drugs as therapies for prevalent, degenerative diseases. The trial is evaluating SOM-401 (K8), a derivative of a nucleoside reverse transcriptase inhibitor.

David Hutton of Ophthalmology Times talks with Eric Donnenfeld, MD about his presentation "Small Aperture IOL for Irregular Corneas" at this year's Hawaiian Eye and Retina 2024 Meeting.
David Hutton of Ophthalmology Times talks with William Trattler, MD, about his presentation "Toric IOL pearls for Correcting Lower Levels of Astigmatism" at this year's Hawaiian Eye and Retina 2024 Meeting.

David Hutton of Ophthalmology Times talks with Andrew Lee, MD, about his presentation "Neuro op mimics of thyroid eye disease" at this year's Hawaiian Eye and Retina 2024 Meeting.

The VISUMAX 800 is the latest generation of Zeiss femtosecond lasers, and offers a reduced laser time when compared to its predecessors.

Student confidence in dealing with ophthalmic conditions is down, deterring them from considering ophthalmology as a career.

According to OKYO Pharma, the first-in-human, randomized, double-masked, placebo-controlled trial of OK-101 “established a clear and informed path for further development in Phase 3 registration trials.”