Oculogenex founder talks about collaboration with NASA and ISS on experiment in macular degeneration
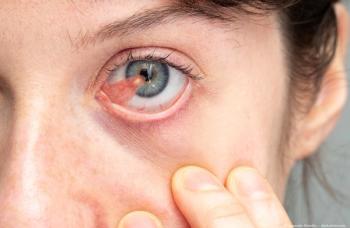
Hema Ramkumar, an ophthalmologist and founder of Oculogenex, sat down with David Hutton of Ophthalmology Times to discuss their connection with NASA and their experiment on macular degeneration-treated mice in space.