
The deal will see MeiraGTx assume development rights to ZipBio’s experimental therapies targeting the complement pathway in geographic atrophy (GA).

The deal will see MeiraGTx assume development rights to ZipBio’s experimental therapies targeting the complement pathway in geographic atrophy (GA).

The 2025 program built on its evolving reputation as a multispecialty educational forum that successfully integrates ophthalmology and optometry, premium CME content, and a family-friendly format to foster professional growth without sacrificing work–life balance.



Andrew G. Lee, MD, and Drew Carey, MD, discuss how optic disc cupping after optic neuritis reflects nerve and ganglion cell thinning, not disease type, helping distinguish it from glaucoma.
Metformin, a common diabetes medication, may be associated with less development of intermediate age-related macular degeneration (AMD).


Family-friendly format empowers continuing education.



NYU Grossman’s Preeya Mehta, MD, and Jonathan S. Williams, MD, sit down with Mount Sinai’s Louis R. Pasquale, MD, to discuss long-term safety and efficacy data from a PreserFlo microshunt trial and the role of microshunts in glaucoma surgery.
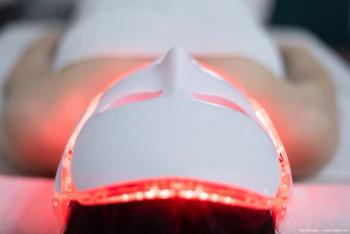
Revisyon is described as a device-led therapy that applies specific wavelengths of low-level LED light to the crystalline lens through a non-invasive, in-clinic procedure.

Proactive strategies enhance comfort, adherence, and long-term glaucoma control

Andrew Tatham, MD, MBA, FRCOphth, FEBO, outlines the role of micro-invasive glaucoma surgery alongside established glaucoma procedures, focusing on safety, patient selection, and future directions.

AI is reshaping clinical practice in retinal imaging and workflow optimization, according to Priv.-Doz. Dr. med. Jan H. Terheyden, FEBO.

As the 2nd International Glaucoma Symposium approaches in Mainz, Germany, Stephan Schulz highlights how the program bridges everyday clinical needs with the latest advances in AI, imaging, and surgery.

Tenpoint Therapeutics’ YUVEZZI, expected in the US in Q2 2026, is the first fixed-dose combination drop designed to improve near vision via pupil modulation with once-daily dosing.


Glaukos noted under the updated labeling, physicians may now re-administer iDose TR more than once in patients who maintain a healthy cornea, as defined by corneal endothelial cell density parameters.

The trial is evaluating OPGx-MERTK gene therapy for MERTK-related retinitis pigmentosa (RP).


The trial will evaluate the safety and effectiveness of the company's titratable glaucoma therapy system, designed to optimize IOP reduction in patients undergoing glaucoma surgery.

According to the company, this meeting helped to provide a “clear path forward” for CBT-004 in its projected phase 3 study


This enables the initiation of the EYETAC phase II clinical trial (NCT07285070) in patients with non-infectious anterior uveitis (NIAU).

From artificial intelligence and IOL innovation to biosimilars, geographic atrophy, and postoperative eye protection, Joshua Mali, MD, FASRS, shares what he believes will define ophthalmology in the year ahead.
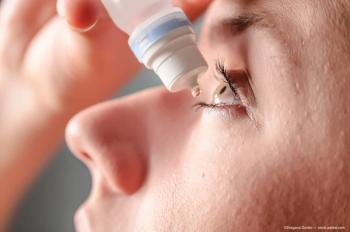
A study reveals that low-dose pilocarpine effectively reduces pupil size in presbyopic eyes with minimal impact on lens thickness and accommodation.
