STAAR releases new date for shareholder meeting

STAAR releases new date for shareholder meeting

New research highlights iron dysregulation's role in dry AMD, suggesting transferrin as a promising treatment to slow disease progression.

Xelafaslatide (formerly ONL1204) is a small-molecule Fas inhibitor designed to protect key retinal cells from cell death that occurs across multiple retinal diseases and conditions such as geographic atrophy.

Andrew G. Lee, MD, and Drew Carey, MD, discuss the case of a patient with persistent papilledema after brain tumor resection due to superficial siderosis.
Faster cutting speeds and ergonomic design streamline workflows and enhance comfort.

The 24-month results of this first-in-human trial supports the potential long-term outcomes of this system to lower IOP.
To celebrate Ophthalmology Times' 50th anniversary, we asked leading experts what the practice would look like today had optical coherence tomography (OCT), one of the biggest innovations in the field, never been invented.

The awards recognize outstanding contributions in 3 categories—Rising Star, Catalyst, and Visionary Leader—honoring individuals driving innovation, inclusion, and impact across ophthalmology.

As a US clinical investigator, Wiley shares his insights on the FineVision HP trifocal lens, highlighting patient visual outcomes, satisfaction, and its impact on cataract surgery practice.
Catch up on news you may have missed at the meeting in Orlando, like the effects of GLP-1 drugs on eye health and a potential sign of cardiovascular disease.

David Eichenbaum, MD, FASRS, recalls an early-career case managing bilateral diabetic macular edema with frequent intravitreal triamcinolone injections—leading to severe steroid-induced glaucoma and valve implants.

Cases show improved clarity, astigmatism masking, and versatility in complex eyes.

Andrew G. Lee, MD, revisits a 1997 case in which recognizing paraneoplastic optic neuropathy led to the diagnosis—and life-saving treatment—of small cell lung carcinoma.

Earlier use of SLT, sustained-release drug delivery, and MIGS is reframing glaucoma care.


Neda Shamie, MD, recounts a humbling experience performing cataract surgery in Honduras using older-generation phaco machines, revealing how technology elevates both skill and patient care.
Roundtable discussion explores surgical and sustained-release options.
Chiu discusses factors affecting adoption of the FDA-approved epi-on therapy, from clinic setup to insurance coverage, while highlighting its safety and efficacy.
REMAIN is the long-term extension of the phase 2b/3 RESTORE trial evaluating MCO-010 in retinitis pigmentosa (RP).

The decision follows results from the Phase 3 STAR trial, among the largest global studies of low-dose atropine in pediatric myopia.

Innovations in surgery, biologics, and AI are shaping the future of eye aesthetics.

Their discussion sheds light on the importance of physician advocacy in ophthalmology—from shaping policy and protecting patients to building community.
HELIOS Phase 1 showed consistent reductions in retinal fluid with OTX-TKI, paving the way for pivotal HELIOS-2 and HELIOS-3 trials.


Inder Paul Singh, MD, shares a formative experience in which a patient’s vision loss—and their unexpected gesture of comfort—shaped his approach to high-risk glaucoma care.
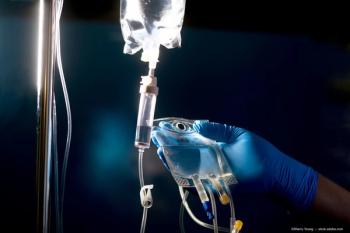
A study reveals that systemic chemotherapy significantly reduces mortality in retinoblastoma, while targeted treatments lower enucleation rates.
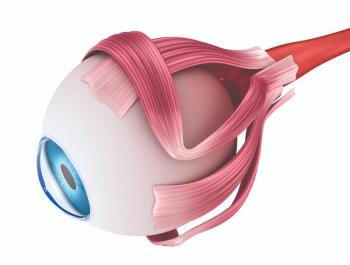
How pupil modulation and pharmacologic targeting shape near vision outcomes.

New research reveals subretinal drusenoid deposits in Black and Hispanic AMD patients may signal higher vascular disease risk

Optogenetics shows promise in improving mobility and object recognition for patients with retinitis pigmentosa and Stargardt disease.

SriniVas R. Sadda, MD, FARVO, recalls a residency case that highlights the transformative impact of OCT and OCT angiography.