
Their machine-learning multistage system predicts the expected disease progression of patients and their VA with age-related macular degeneration (AMD), diabetic macular edema (DME), and retinal vein occlusion (RVO).

Their machine-learning multistage system predicts the expected disease progression of patients and their VA with age-related macular degeneration (AMD), diabetic macular edema (DME), and retinal vein occlusion (RVO).

The study showed that a 12-day treatment of KIO-101 topically at multiple doses was well tolerated in healthy volunteers and patients with inflammation of the eye.

Results show Eylea HD extended dosing regimens were non-inferior to aflibercept 2 mg injection (Eylea) for both the treatment of wet age-related macular degeneration (wAMD) and diabetic macular edema (DME).

TL-925 is a first in class, topical Bruton’s tyrosine kinase (BTK) inhibitor that prevents mast cell activation and degranulation, and also blocks the cytokine-driven inflammatory response, and restores lacrimal unit homeostasis, offering the potential for disease modification
Jeff Todd, president and CEO of Prevent Blindness, and astrophotographer Jon Carmichael sat down with David Hutton of Ophthalmology Times to talk about how ophthalmologists can help make sure patients are viewing the eclipse safely as well as proper ways to view/photograph the event without damaging your eyes or equipment.

According to the company, the milestone underscores Vivity’s wavefront-shaping technology, which simplifies presbyopia correction for surgeons and patients.
Study concluded that the short-term results of LBN to treat mild-to-modern OAG and OHT was safe and efficacious.

The company has divested its Coherus Ophthalmology subsidiary to Sandoz, Inc. for upfront all-cash consideration of $170 million.
Eyenovia acquired the US commercial rights for the drug last August from Formosa Pharmaceuticals.

ChatGPT-4 (OpenAI) had an overall “fair” performance when answering multiple-choice ophthalmic questions unrelated to multifocal imaging.

Andrew G. Iwach, MD, recounts two personal life experiences that underscore the significance of patient-centered care in glaucoma management.

Early detection of the disease is key to optimal patient outcomes

After completing his residency at the Wilmer Eye Institute and a fellowship at NIH, Walter J. Stark, MD, returned to Wilmer for a 40-year career as a surgeon, teacher, researcher, and Boone Pickens Professor of Ophthalmology and chief of the Cornea Division.

During the 2024 American Glaucoma Society meeting held February 29 to March 3 in Huntington Beach, California, Nixoc SA presented posters outlining data on NCX 470.

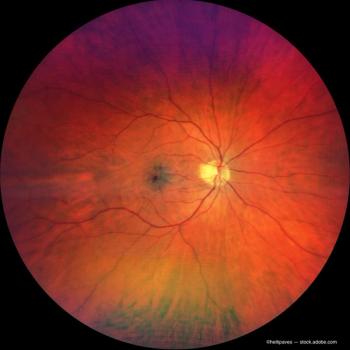
Mobile cameras allow University of Colorado ophthalmology residents working in hospital settings to conduct more comprehensive and efficient patient evaluations.
David Sola-Del Valle, MD, sat down with David Hutton of Ophthalmology Times to discuss his talk at the annual American Glaucoma Society meeting held in Huntington Beach, about the importance of bridging the language barrier in patients who may not be fluent in English.

April Maa, MD, sat down with David Hutton of Ophthalmology Times to discuss her presentation at the annual American Glaucoma Society meeting held in Huntington Beach, about tips and tricks for implementing telehealth into ophthalmology.

Repetitive exercises before drop instillation, may decrease difficulty and improve the successful instillation of eye drops.

In the trial, PL9643 failed to meet co-primary and secondary endpoints.

In ophthalmology, women continue to take on leadership roles and make a difference in the profession.

Michael Greenwood, MD, discusses the safety of laser refractive surgery for patients at high risk for glaucoma, focusing on the need for thorough pre-surgery assessment, careful monitoring of eye pressure during and after the procedure, and awareness of prolonged steroid use following PRK.

Steven R. Sarkisian, MD, sat down with David Hutton of Ophthalmology Times to discuss his poster about the Travoprost intraocular implant in phakic eyes from the American Glaucoma Society meeting held in Huntington Beach
I. Paul Singh, MD, sat down with David Hutton of Ophthalmology Times to discuss his poster "Travoprost Intraocular Implant Demonstrates Efficacy in Diverse Subpopulations of OAG and OHT Subjects," from the American Glaucoma Society meeting held in Huntington Beach

The supplement is specifically crafted to promote healthy intraocular pressure (IOP), nurturing the well-being of retinal ganglion cells, and sustain the health of the retinal nerve fiber layer while boosting memory and concentration.

Lowe syndrome, a genetic disease, affects about 1 in 500,000 people in the general population.
Leon Herndon, MD, President of the American Glaucoma Society, sat down with David Hutton of Ophthalmology Times to discuss the AGS meeting held in Huntington Beach and what attendees can expect as well as new and exciting information to come from the meeting.