
Lens combines diffractive multifocal and extended depth-of-focus properties.

Lens combines diffractive multifocal and extended depth-of-focus properties.
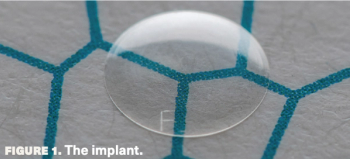
New acrylic implant provides option for patients with endothelial dysfunction.
Product stimulates basal tear production, restores tear film homeostasis, and relieves symptoms.