Interventional Glaucoma
Latest News
Latest Videos

CME Content
More News

iStent implantations increased sharply, whereas trabeculectomies saw a decline.


Researchers note work may lead to targets for conventional outflow pathway therapies.

According to the company, the Denali trial is evaluating NCX 470 safety and efficacy in patients with open-angle glaucoma or ocular hypertension and topline results continue to be expected in H2 2025

Therapeutics can prevent vision loss and neurodegeneration in patients.
Dr. Khouri discusses the MIGS procedures he performs in his practice and his approach to selecting the most appropriate procedure for each patient.

Dr Khouri presents a case of a glaucoma patient who underwent multiple SLT procedures and would be a good candidate for MIGS.

Dr Khouri discusses selective laser trabeculoplasty (SLT) as a first-line treatment for glaucoma and identifies patients who would be good candidates for this procedure.

Dr Albert S Khouri, MD presents a case of a glaucoma patient and discusses his approach to medical management of glaucoma.
Danny A. Mammo, MD, sat down with Sydney Crago, Assistant Managing Editor of Modern Retina, to discuss his presentation on intraocular pressure outcomes following suprachoroidal triamcinolone acetonide in patients with glaucoma at the American Society of Retina Specialists meeting held in Stockholm, Sweden.
Researchers indicate that minimally invasive treatment with NurExon Biologic’s ExoPTEN showed functional restoration of damaged eyes to healthy levels in animals.

According to the company, FYSX is the first non-pharmacological, non-surgical treatment designed to lower IOP for glaucoma patients.
Technology offers increased usability, safety, and accuracy in MIGS procedures.

The deal included a total upfront consideration of $81 million, including a cash payment of approximately $65 million.

The patient-facing brochure serves as a vehicle to help ECPs review the solution’s information and prescription filling process with patients during and after an office visit, and the website serves as an additional resource and reference once the patient returns home.

A team of Korean researchers has examined data from the Korean National Health Information Database to determine whether subjects with allergic diseases exhibited a higher incidence of primary open-angle glaucoma compared to a control group.

All R&D for the drug will stop after the Phase 2a clinical trial failed to meet its primary endpoint of lowering IOP in patients with primary open-angle glaucoma.
McDonnell stays focused on helping the next generation of ophthalmologists launch their careers in academics.

Lorraine Provencher, MD, reviews the NOVA trial which measured and validated the sensitivity of a novel VR-based wearable headset for visual field testing in glaucoma.

The company’s KDB GLIDE is indicated for the excision of trabecular meshwork tissue to reduce intraocular pressure in adult patients with primary open-angle glaucoma during cataract surgery or as a stand-alone procedure.

The campaign will encourage, educate and empower the public to include vision and eye health as a part of their healthcare by asking them to share how eye care services and exams improved their vision and their daily lives.

Alcon agreed to acquire Belkin Vision and the Belkin Vision Eagle and plans to provide additional details when the deal is closed.
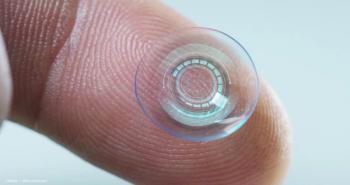
A team of Chinese researchers have been working on a way to continuously and more comfortably detect these tiny fluctuations in pressure, such as contact lenses that transmit signals to receptor glasses.


A singular celebration of science over the decades