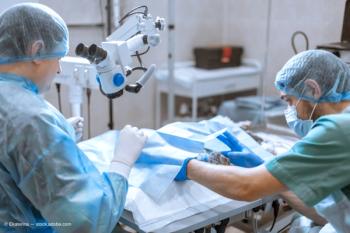
Bausch + Lomb’s ClearVisc dispersive ophthalmic viscosurgical device has received FDA approval for use in ophthalmic surgery.

Bausch + Lomb’s ClearVisc dispersive ophthalmic viscosurgical device has received FDA approval for use in ophthalmic surgery.

The FDA has approved loteprednol etabonate ophthalmic suspension 0.25% for short-term treatment of the signs and symptoms of dry eye disease.

This action will result in 2 separate companies,


Provider portal features an updated enrollment form, and potential for a 60-second benefit verification.

The company promotes two and adds another employee as it names three vice presidents.

Pseudophakic patients may benefit from a fluocinolone acetonide intravitreal implant (Iluvien, Alimera Sciences), the company tells a United Kingdom committee.

A cellular-level adaptive optics retinal camera (rtx1, Imagine Eyes) has received CE mark approval in the European Union.

A new sealant (ReSure, Ocular Therapeutix) will be studied for clear corneal incision closure after cataract extraction or IOL placement.

Improved efficacy and dosing convenience are the aims of a new drug delivery system incorporating a cationic polymer and formulated with ketorolac.

New data presented at the European Society of Retina Specialists meeting suggest that ranibizumab is effective in patients with AMD, DME, and RVO.

Newly published research gives ophthalmologists a reason to encourage their adult patients with visual field loss in both eyes to be more physically active.

Researchers have identified the mode of death of cone photoreceptor cells in an animal model of retinitis pigments (RP).

Glaucoma and age-related macular degeneration (AMD) research grants have been awarded to Wilmer Eye Institute by the American Health Assistance Foundation.

The Welch Allyn facility in Beaverton, OR, earned Leadership in Energy and Environmental Design (LEED) certification from the U.S. Green Building Council.

William M. Moore has been named interim president and chief executive officer of Iridex Corp. Moore succeeds Dominik Beck.

Implantable contact lens and preloaded IOL manufacturing will move to California in a consolidation project now under way at STAAR Surgical.

Glaucoma may be a significant predictor of depression, according to research published in the American Journal of Ophthalmology.

The FDA approved OptiMedica's Catalys Precision for creating single-plane and multi-plane arc incisions in the cornea during cataract surgery.

Ocular Therapeutix Inc. is initiating a phase II clinical trial of OTX-TP2, a 2-month travoprost punctum plug for treating ocular hypertension and glaucoma.

Gevokizumab, an IL-1 beta-modulating antibody from XOMA Corp., has received FDA orphan drug designation for the treatment of several types of non-infectious uveitis.

Ophthalmologists will have a new way to learn about advances in laser surgery next year, thanks to a $1 million donation made to the American Academy of Ophthalmology.

The Foundation Fighting Blindness is funding projects related to age-related macular degeneration, retinitis pigmentosa, Leber's congenital amaurosis, and Usher syndrome.

The Joint Commission on Allied Health Personnel in Ophthalmology (JCAHPO) and Pearson VUE partner to provide ophthalmic medical techs access to simulated exams.

Insight Software, creators of My Vision Express, has been ranked by Inc. magazine as one of the nation's fastest-growing private companies.

Patient enrollment has begun in a phase III clinical trial of a low dose of bromfenac for pain and inflammation reduction after cataract surgery.

Prevent Blindness America (PBA) has designated September as Sports Eye Safety Awareness Month.

Compared to an average of all physicians, ophthalmologists fared slightly better in a survey of physician burnout.
Published: June 18th 2020 | Updated:

Published: August 22nd 2012 | Updated:

Published: August 22nd 2012 | Updated:

Published: August 22nd 2012 | Updated:

Published: August 22nd 2012 | Updated:

Published: August 22nd 2012 | Updated: