
OCT and OCTA imaging were especially valuable in this patient population because the images extended the current definition of sickle cell retinopathy.

OCT and OCTA imaging were especially valuable in this patient population because the images extended the current definition of sickle cell retinopathy.

Light-adjustable lens in patients with previous RK: Improved visual and refractive outcomes after cataract surgery.
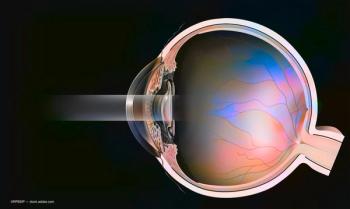
Physician notes endothelial cells play key role in hydration, transparency of cornea.

New drugs are now in the pipeline to focus on treatment of the disease.

Technology allows partitioning of sequencing reads into 2 parental genome data sets.
Physician discusses 5 keys to achieve optimal results for patients

Collaboration between ophthalmologists, optometrists can boost early detection.

Developing technology may offer marked improvements in surgical precision
Polish researchers found positive bacterial cultures in premature infants correlated with severe retinopathy of prematurity (ROP), suggesting a potential prognostic marker for early ROP development.

World Sight Day on October 12 highlights the vital role of eye care in workplaces. The International Agency for the Prevention of Blindness urges employers to prioritize eye health, aiming to enhance well-being, safety, and productivity.

Educating patients about the importance of consistent care is key.
A rapid lateral-flow device shows high sensitivity and specificity in identifying Aspergillus species in corneal samples, aiding in diagnosing microbial keratitis. The device, coupled with ratiometric analysis, proves robust and cost-effective, potentially revolutionizing MK diagnosis, especially in low-resource settings

Efficacy of treatment option is also confirmed in pediatric population in India.

Clinicians must screen, diagnose, and manage for effective treatment.

Leaders must confront issues perpetuating unequal access, unfair practices.

Patients with HTG had significantly fewer mtDNA copies per nuclear DNA.

When smokers used both cigarettes and e-cigarettes, studies showed additional detrimental findings, such as lower general health, increased sleep latency, and higher vascular disorder risk
The 6-month-old boy’s dark brown eyes turned deep blue after he was treated for COVID with favipiravir.
Researchers find gray GA lesions had the highest cross-modality differences

The investigators performed a retrospective cohort study of patients from 10 US ocular inflammatory disease (OID) subspecialty practices.

Treating the meibomian glands and lifestyle changes are among some of the treatment methods.

Currently, miotics are taking center stage for treating presbyopia.


Henriquez pointed out that waiting for keratoconic progression runs the inherent risk that keratoconus will progress rapidly and affect the planned initial treatment protocol.

Lead author Sean Paul, MD, reports radiofrequency treatment in conjunction with expression of the meibomian glands reduces the signs and symptoms of dry eye disease.

Researchers have identified markers that indicate the presence of Parkinson’s disease in patients an average of 7 years before the disease presents clinically.

Data may inform clinical associations, mechanistic research, trial design.

Clinicians should be alert to the fact that while artificial intelligence (AI) is capable of generating ideas and references, it is crucial to thoroughly vet and fact-check any medical research content that AI produces.

Without treatment, severe visual impairment can occur; the first-line treatment for these cases is anti-vascular endothelial growth factor therapy (VEGF).
Researchers combine blue reflectance, red/green 200° ultrawidefield images