
According to the company, the study has now reached its goal of 300 patients enrolled to evaluate ILUVIEN as a first line, baseline therapy for DME.

According to the company, the study has now reached its goal of 300 patients enrolled to evaluate ILUVIEN as a first line, baseline therapy for DME.

Instrumentation recreates properties of the myopic eye to test lenses that prevent visual decline.

According to the company, study results show sustained reductions in both IOP and glaucoma medication use in mild-moderate primary open angle glaucoma patients treated with a procedure enabled with the OMNI Surgical System technology.

According to the company, the Phase 1 trial is a multicenter, open-label, dose-escalation safety clinical trial. Up to 24 subjects will receive a single periocular injection and will undergo monthly evaluation for up to 6 months to assess safety, tolerability, and efficacy measured by best corrected visual acuity.

According to the company, the DIAMOND trial in diabetic macular edema with topical OCS-01 met its stage 1 objective of validating the loading and maintenance dosing regimen designed to optimize OCS-01 efficacy potential with robust statistical significance.
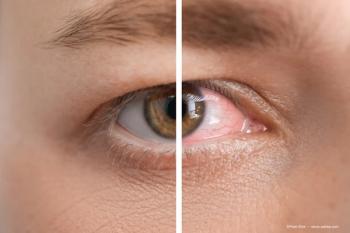
The company noted tanfanercept did not demonstrate statistical significance in either of the primary outcome measures of improvement in central corneal staining score or in improvement in Eye Dryness Score assessed at week 8 in subjects with dry eye disease, compared to vehicle. However, tanfanercept did demonstrate a highly statistically significant improvement on one of the secondary outcome measures, Schirmer testing of tear volume.
Ophthalmology Times reached out to members of its editorial advisory board along with ophthalmologists who specialize in dry eye for their comments on what this approval means for their patients with dry eye.

According to the companies, MIEBO is the first and only prescription eye drop approved for dry eye disease that directly targets tear evaporation, based on consistent results from a pair of pivotal Phase 3 trials.

During the 26th annual meeting of the American Society of Gene & Cell Therapy, taking place through Saturday in Los Angeles, California, the company is delivering several presentations highlighting its AAV capsids and gene constructs.

The American Academy of Ophthalmology Truhlsen-Marmor Museum of the Eye, a free public museum dedicated to the science of sight, will present Decoding the Eye.: Signs and Symbols.

The company noted YUTIQ is used for the treatment of chronic non-infectious uveitis affecting the posterior segment of the eye, and is the same as Alimera’s ILUVIEN.

In a presentation at the American Society of Cataract and Refractive Surgery meeting in San Diego, Kenneth A. Beckman, MD, FACS, discussed the current status of corneal crosslinking with some emerging treatments, including epi-on crosslinking.

Earlier this year, a federal civil jury concluded that the defendants violated the False Claims Act and the Anti-Kickback Statute by paying kickbacks to ophthalmic surgeons to induce their use of the defendants’ products in cataract surgeries reimbursed by Medicare.

Diana V. Do, MD, presented details of the PHOTON study at the Association for Research in Vision and Ophthalmology annual meeting, held recently in New Orleans, highlighting the safety and efficacy of 8 milligrams of aflibercept.

In November 2022, doctors in Cleveland diagnosed a patient with a corneal ulcer with a Pseudomona aeruginosa infection. The patient acquired the infection from tainted EzriCare eye drops months before the CDC’s February 2023 warning.

According to the company, its new 20,000-square-foot facility in Hayward, California, includes cGMP suites, developmental and testing laboratories, equipped with single use bioreactors.
Steven Greenstein, MD, sat down with David Hutton, Managing Editor, Ophthalmology Times®, to discuss his presentation about the 10-year follow-up for collagen cross-linking and keratoconus ectasia.

According to the U.S. Attorney’s Office, District of Rhode Island, Paul S. Koch, MD, former owner of several ophthalmology practices in the state, agreed to settle civil allegations he paid kickbacks to optometrists who referred patients to his practices for cataract surgeries.

The goal of the project is to encourage patients with Demodex blepharitis to visit an ophthalmologist.

The scanning laser features a wireless user interface, no external fiber and utilizes MEMS technology.

Vivek Srinivasan, PhD, sat down with David Hutton, Managing Editor, Ophthalmology Times®, to discuss his presentation about using visible light, OCT to image the retina at this year's ARVO meeting.

If approved, RVO would be the third indication for Vabysmo in addition to wet age-related macular degeneration and diabetic macular edema.
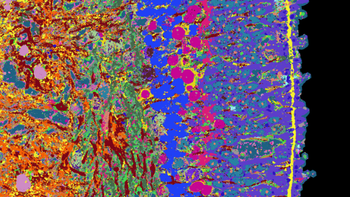
A team of researchers has now developed an approach to gather and compile a great deal of information about organoids and their development. The team applied its approach to the organoids of the human retina, which they derived from stem cells.

According to researchers, these chronic, progressive retinal diseases, including retinitis pigmentosa, arise from genetic and environmental disruptions of cellular and tissue stability.
According to the organization, the trip marks the 12th training project in Vietnam for the Flying Eye Hospital, including one virtual project on Cybersight, Orbis's telemedicine platform.

At the American Society of Cataract and Refractive Surgery annual meeting in San Diego, data was presented from the STORM study that demonstrate vision quality to be a major driver of the patient decision to undergo surgery.

According to the company, the announcement represents the first FDA-approved, fixed-combination of tropicamide and phenylephrine for mydriasis.

According to the company, a PDUFA date has been set for December 22, 2023.

Scientists at Medical College of Georgia demonstrated in their animal model of retinopathy of prematurity that the small molecule K604, which is being explored in cancer and Alzheimer’s, can block the development of leaky, obstructive blood vessels in the retina, tamp down inflammation and enable more normal blood vessel growth

Alcon presented details from a new study at the American Society of Cataract and Refractive Surgery’s annual meeting taking place in San Diego, California. The investigator-initiated study led by J. Morgan Micheletti, MD, demonstrated that Clareon and Eyhance monofocal IOLs provide similar range of vision, including distance and intermediate visual acuity.