According to the company, if approved, OCS-01 has the potential to become the first topical eye drop and non-invasive treatment option for DME.

According to the company, if approved, OCS-01 has the potential to become the first topical eye drop and non-invasive treatment option for DME.

Physicians from the Harvard Medical School and the Kresge Eye Institute at Wayne State University in Detroit recently published details from the case.

According to the company, the NORSE EIGHT study is set to kick off during the first quarter of 2024 and the Biologics License Application in the US is likely by the end of 2024.

Ocugen says the Regenerative Medicine Advanced Therapy designation will "help expedite the development of new regenerative medicines."

The companies announced they will team up to enhance patient care and workflow efficiencies with a comprehensive dry eye assessment and relief platform.

Marc Gleeson, CEO of Azura, sat down with David Hutton, Managing Editor at Ophthalmology Times to discuss the recent positive results from the Phase 2 clinical trial of AZR-MD-001 in patients with contact lens discomfort.
NCX 470, a novel nitric oxide-donating bimatoprost eye drop, is the company’s lead product candidate in Phase 3 clinical development for IOP lowering in patients with open-angle glaucoma or ocular hypertension.
According to the company, the study met its primary endpoint and showed significant and clinically meaningful improvements in multiple symptoms of contact lens discomfort and signs of concomitant meibomian gland dysfunction.

A genome topology map of human retina development lays the foundation for understanding diverse clinical phenotypes in simple and complex eye diseases.

A team of researchers from Tokyo Medical and Dental University have developed models based on machine learning that predict long-term visual acuity in patients with high myopia, one of the top three causes of irreversible blindness in many regions of the world.
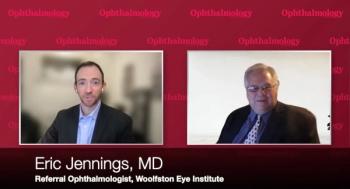
Eric Jennings, MD, from Woolfson Eye Institute, discusses the SMARTCataract cloud-based app from Alcon and its implementation where he practices with David Hutton, Managing Editor, Ophthalmology Times.

According to the company, the acquisition will extend its leadership in ophthalmic medical devices market and expands its position in the vitreo-retinal surgery segment.

The research marks the first attempt at integrating a photoactivatable anti-angiogenic agent with a photosensitizer into a single nanoformulation for AMD treatment.

Glaukos Corp's iDose TR has received FDA approval for the reduction of IOP in patients with ocular hypertension (OHT) or open-angle glaucoma (OAG) following a new drug application (NDA) submission.

Randomization has been completed and, according to the company, topline data is expected during the third quarter of 2024.

According to the company, OCU410 is a modifier gene therapy product candidate being developed for dry AMD

A Q-switched, 532 nm-wavelength, frequency-doubled Nd:YAG laser, the Eagle is intended for use in performing selective laser trabeculoplasty.

According to the company, the device is designed to offer a non-surgical solution for patients suffering from keratoconus, corneal irregularities, photophobia, and presbyopia.

According to the company, its RELIEF Phase 2b trial will evaluate the efficacy and safety of licaminlimab in moderate-to-severe dry eye disease, and further explore the potential of a genetic biomarker. Topline results are expected in mid-2024.

Fish have the built-in ability to regenerate retinal neurons by turning another retinal cell type called Müller glia into neurons. Researchers have been able to coax the human Müller glia into changing identity in the laboratory, which could serve as a potential source of new neurons to treat vision loss.

According to the company, ATSN-101 continues to demonstrate clinically meaningful improvements in vision at the highest dose and is well-tolerated 12 months post-treatment.

The company announced key secondary endpoints were achieved with both EYP-1901 doses. These include a more than 80% reduction in treatment burden, with nearly two-thirds of eyes supplement-free up to 6 months.
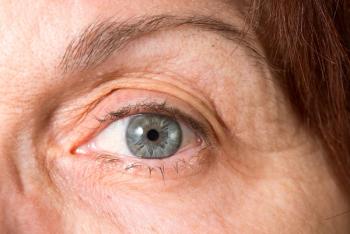
Prevent Blindness is providing free geographic atrophy educational resources for patients, care partners and healthcare professionals, including a new episode of its Focus on Eye Health Expert series.

The center includes about 30,000 square feet of space for rapid prototyping of new product designs, enhance manufacturing capabilities, and the expansion of research and development capabilities.

Benjamin Y Xu, MD, spoke with the Ophthalmology Times team about the real-world safety analysis of patients treated with intracanalicular dexamethasone insert using IRIS Registry at this year's American Academy of Ophthalmology meeting.

Rupa Wong, MD, spoke with the Ophthalmology Times team about Luminopia, the first FDA-approved digital therapeutic for amblyopia at this year's American Academy of Ophthalmology meeting.

The company announced a resubmission of the ONS-5010 BLA on track for the end of calendar year 2024, pending final agreement on a clinical trial protocol with the FDA and successful completion of the required additional clinical trial.

According to the company, its Zilia Ocular FC system is designed for assessing ocular biomarkers.

Earlier this year, Harrow acquired the US commercial rights to Triesence. Aside from the transfer of the Triesence NDA ahead of the date previously agreed to, all other acquisition terms remain unchanged.

The Phase 2 study is evaluating the efficacy and safety using 2 concentrations of SBI-100 OE vs. placebo, dosing twice a day for 14 days.