Conference Coverage
about 1 month ago
IRIS Registry data mined for understanding of pediatric NKabout 1 month ago
Reflections from the floor: AAO memories that shaped careersLatest News

MeiraGTx Licenses complement-targeted geographic atrophy program from ZipBio

Looking back at the 2025 EnVision Summit

Last year in glaucoma at EnVision Summit 2025

China's NMPA approves ZEISS ARTEVO 750 and ARTEVO 850 ophthalmic surgical microscopes for clinical use
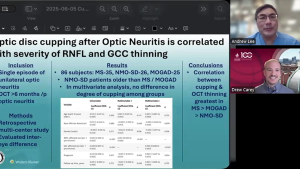
NeuroOp Guru: Understanding optic disc cupping after optic neuritis

Shorts










Ophthalmology Times Digital Edition

Podcasts
Continuing Medical Education
All News
Metformin, a common diabetes medication, may be associated with less development of intermediate age-related macular degeneration (AMD).


Family-friendly format empowers continuing education.



NYU Grossman’s Preeya Mehta, MD, and Jonathan S. Williams, MD, sit down with Mount Sinai’s Louis R. Pasquale, MD, to discuss long-term safety and efficacy data from a PreserFlo microshunt trial and the role of microshunts in glaucoma surgery.
Revisyon is described as a device-led therapy that applies specific wavelengths of low-level LED light to the crystalline lens through a non-invasive, in-clinic procedure.

Proactive strategies enhance comfort, adherence, and long-term glaucoma control

Andrew Tatham, MD, MBA, FRCOphth, FEBO, outlines the role of micro-invasive glaucoma surgery alongside established glaucoma procedures, focusing on safety, patient selection, and future directions.

AI is reshaping clinical practice in retinal imaging and workflow optimization, according to Priv.-Doz. Dr. med. Jan H. Terheyden, FEBO.
As the 2nd International Glaucoma Symposium approaches in Mainz, Germany, Stephan Schulz highlights how the program bridges everyday clinical needs with the latest advances in AI, imaging, and surgery.

Tenpoint Therapeutics’ YUVEZZI, expected in the US in Q2 2026, is the first fixed-dose combination drop designed to improve near vision via pupil modulation with once-daily dosing.