Publication
Article
Digital Edition
Tapping metabolomics for timely detection of glaucoma cases
Author(s):
Push to identify biomarkers for earlier diagnosis, new therapeutic targets.
(Image Credit: AdobeStock/MQ-Illustrations)

Reviewed by Neeru Vallabh, PhD
The number of individuals worldwide who are affected by glaucoma and who will be in the future is burgeoning. The current push is to identify biomarkers for earlier diagnosis, both to determine those at higher risk of progression and to provide new therapeutic targets, according to Neeru Vallabh, PhD, clinical senior lecturer in the Department of Eye and Vision Science, Institute of Life Course and Medical Sciences, University of Liverpool, and an Honorary Consultant Ophthalmologist for Glaucoma at St Paul’s Eye Unit, Liverpool University Hospitals NHS Foundation Trust, United Kingdom.
“The current diagnostic challenges are the asymptomatic nature of glaucoma and the limitations of the traditional diagnostic methods,” Vallabh said. “While genetic studies have been hugely successful in improving our understanding of the disease, the mechanisms through which genetic factors interact with nongenetic factors and lead to glaucoma are not well understood.”
Vallabh pointed out the need for a transition from reactive medicine to predictive, preventive, and personalized medicine.1 This transition may be through metabolomics.
Metabolomics defined
Metabolomics is the qualitative and quantitative analysis of low-molecular-weight small molecules2 (ie, metabolites with molecular weights under 1500 Da). These include amino acids, carbohydrates, nucleosides/nucleotides, tricarboxylic acid, intermediates, and lipids. The human metabolome, defined as the complete set of small molecule substances, is now thought to contain more than 110,000 metabolites, but many have yet to be identified. Metabolomics, Vallabh explained, reflects the physiological or pathological state of a cell or tissue; provides information about interactions between genetics, environment, and lifestyle; and is closely related to the phenotype in multifactorial disease.3 The metabolites in humans, which are the downstream end products of genes, RNA, and proteins and their interaction with the environment, are critical for the growth and maintenance of cells and tissues in the body and reflect an individual’s functional states.
Metabolomics studied
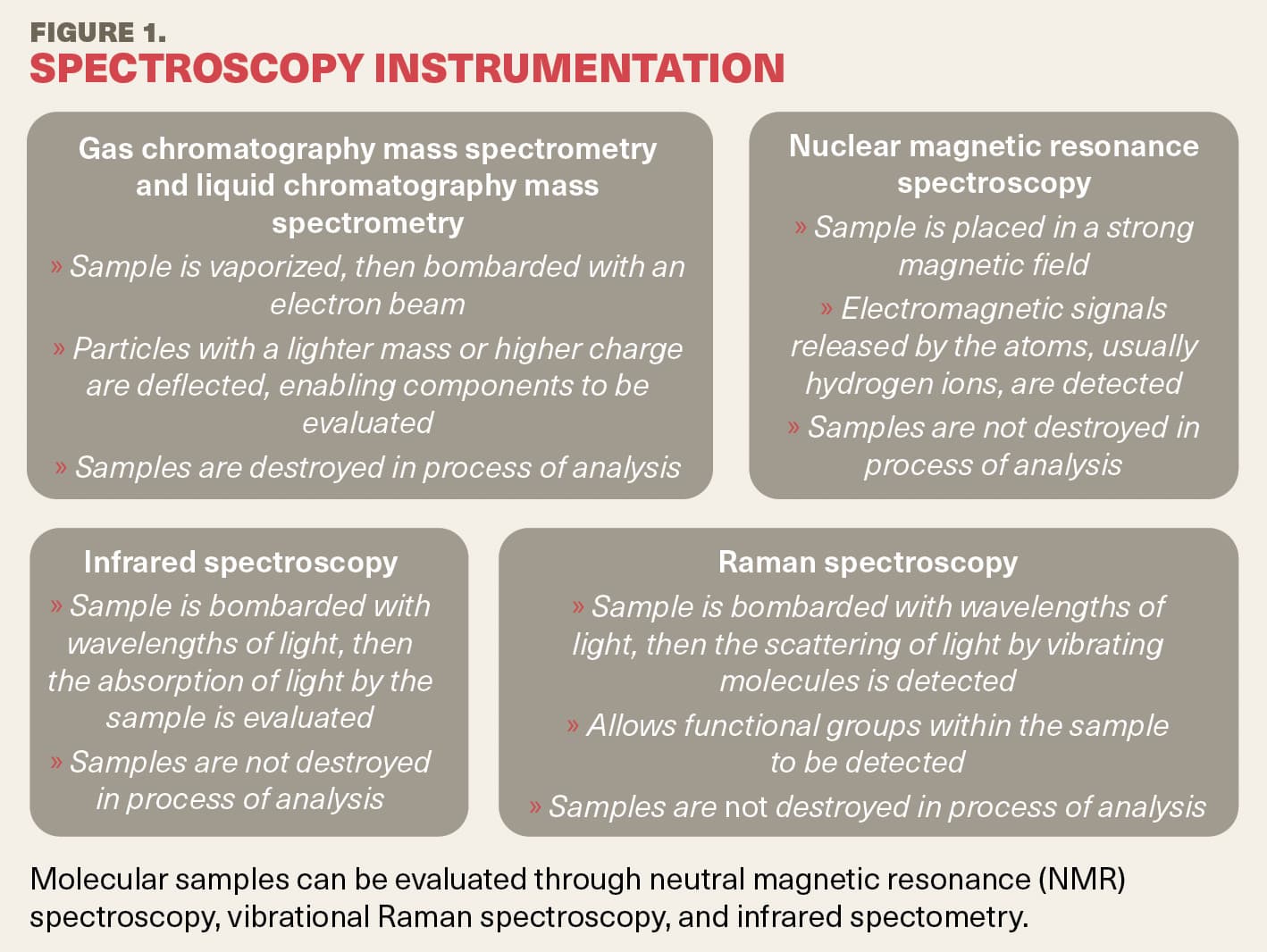
Mass spectrometry can be used to study metabolomics, and there are 2 choices within that route: gas chromatography mass spectrometry or liquid chromatography mass spectrometry4,5 (Figure 1). Using this technique, Vallabh explained, a sample is vaporized (destroyed), and an electron beam bombards the vapors and converts them into charge particles deflected by a magnetic field. The particles with a lighter mass are deflected more. A detector measures the mass charge ratio, and by doing so, the components of the sample can be evaluated.
In another technique, nuclear magnetic resonance spectroscopy, the samples are not destroyed. The samples are placed within a strong magnetic field, and the electromagnetic signals released by the atoms, usually hydrogen ions, are detected.6,7 In vibrational spectroscopy, another technique, the samples are bombarded with a specific wavelength of light. If the scattering of the light by the vibrating molecules is evaluated, this is termed Raman spectroscopy and allows the detection of the functional groups in the sample, whereas if the absorption of the sample by infrared light is evaluated, this is called infrared spectroscopy.6,7
Metabolomics workflow
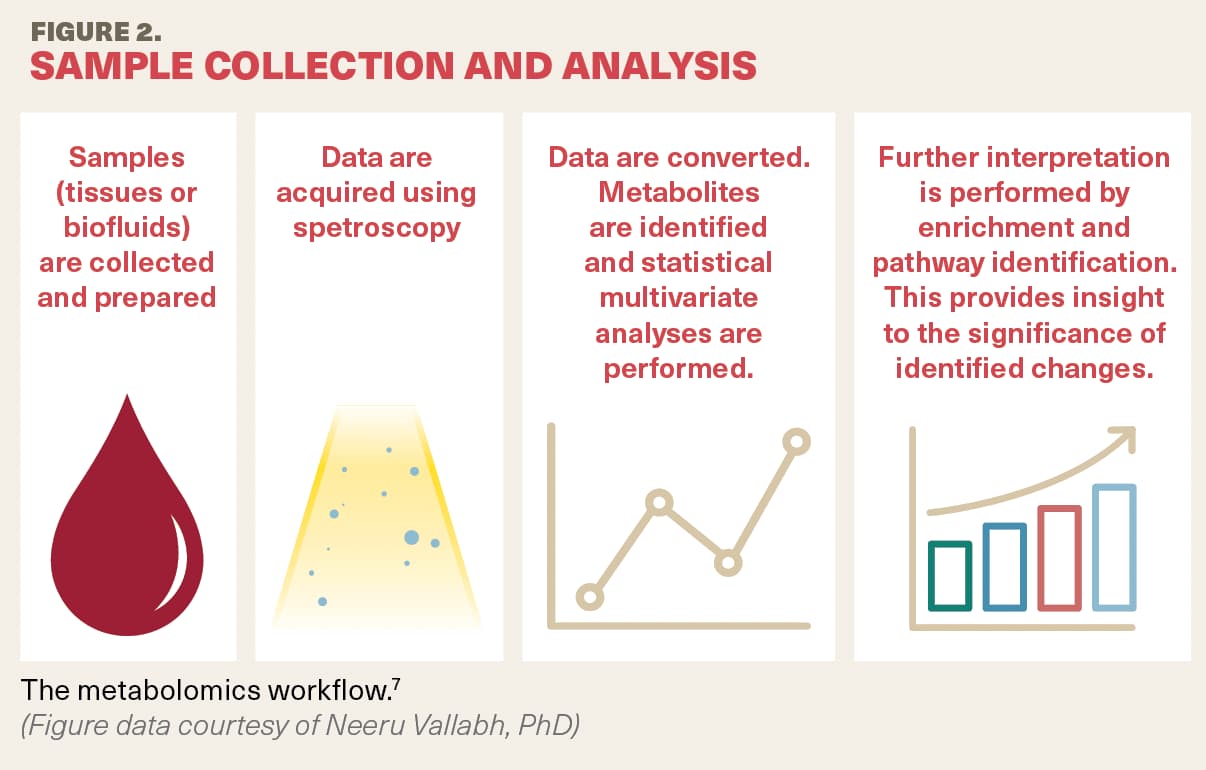
This process involves several steps.8 The sample, which can be tissue or biofluids, is collected and prepared. Data then are collected via the spectroscopy method of choice. The analysis is conducted, normalization and metabolites are identified, and statistical multivariate analyses are performed. Finally, further interpretation is made by enrichment and pathway identification to understand the significance of the changes that have been identified, she described (Figure 2).
Metabolites in glaucoma
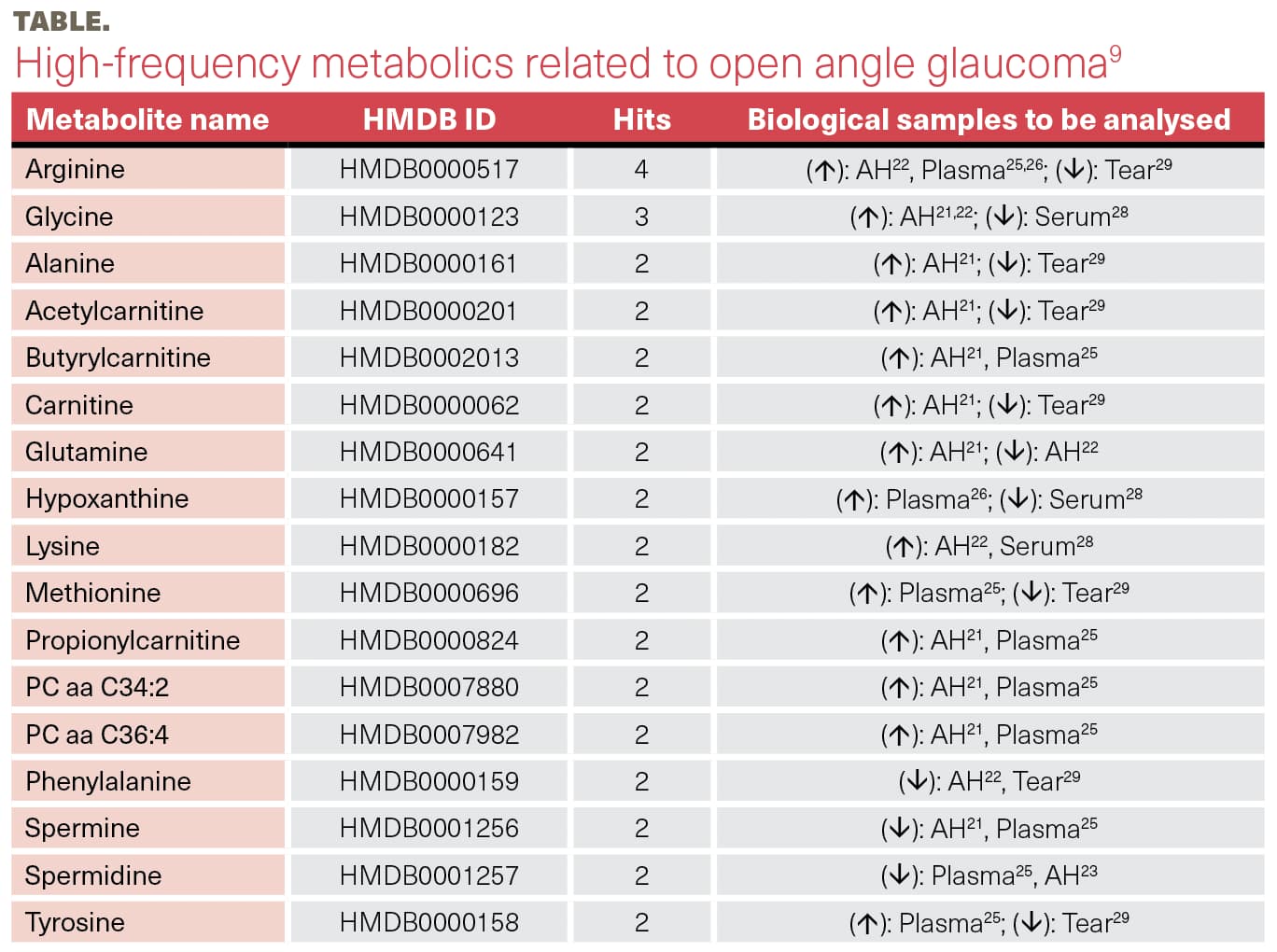
Vallabh reported that in open-angle glaucoma, certain metabolites have a higher frequency, including amino acids, such as arginine and glycine.9 Spectroscopy of aqueous humor, plasma, serum, and tear samples has been performed, but the results in the aqueous humor differ from those in the serum and tears.
She explained that metabolic alterations in glaucoma include amino acid dysregulation, vitamin metabolism, fatty acid oxidation, and glutaminolysis. A systemic review in 202110 reported 24 significantly enriched metabolism pathways.
In glaucoma, the disturbed pathways involve lipid metabolism and the carnitine shuttle pathway, with increased and decreased levels of metabolites and inconsistent changes. “These altered metabolites can impact the mitochondrial fatty acid metabolism and the vulnerability of the retinal ganglion cells,” she stated (Table).
She cited a study of a rat model in which IOP elevations were induced.11 Twenty-five metabolites were found to be significantly altered after 3 days of induced IOP increases in the rat model. These changes were dominated by metabolites that implicate oxidative stress, increased metabolic need, and dysfunction of metabolite production.
Pretreatment with oral nicotinamide for 7 days before the induced IOP increase showed that nicotinamide treatment prevented these changes. Future research will evaluate the impact of nicotinamide treatment on humans and metabolite regulation.
Vallabh and colleagues performed a small pilot study using vibrational spectroscopy to evaluate differences between 10 glaucoma and 10 control aqueous humor samples from the Liverpool Research Eye Bank. The samples were assessed using infrared spectroscopy to measure molecular vibrations.
The results showed that 77.7% of control samples were correctly identified as a control, whereas 78.1% of glaucoma samples were correctly assigned as glaucoma. The gas chromatography-mass spectroscopy results from some of these samples indicated that the signatures observed were mainly associated with amino acids, fatty acids, and some monosaccharides.
The largest-scale metabolite study in glaucoma was a case control study12 that evaluated plasma metabolite profiles in primary open-angle glaucoma. This was performed in 3 US groups that evaluated 369 metabolites using liquid chromatography-mass spectroscopy; the UK biobank study measured
168 metabolites using magnetic resonance spectroscopy. The study found that diglycerides and triglycerides were adversely associated with glaucoma and may play an important role in glaucoma pathogenesis.
Two in vivo assessments of metabolomics13,14 assessed the metabolomics changes. Sentosa et al13 developed a custom-built combined infrared fundus imaging, swept-source optical coherence tomography, and Raman spectroscopy instrument for use in rat eyes. They found that Raman spectra could be observed, although there was a low signal collection efficiency. Cakir et al14 also developed a Raman spectroscope to image the retina and evaluate the oxidative stress status of mitochondria in hemoglobin in mice with induced high IOP.
Moreover, Vallabh pointed out that these are early models, and our understanding of what these spectra or peaks mean at this stage is limited. However, this demonstrates the potential of harnessing our imaging techniques to evaluate metabolite changes in vivo.
Metabolomics limitations
Ongoing research has associated the different metabolites and their corresponding pathways that are altered in glaucoma. However, there are downsides. These include cost, sample size, sample types, limited number of metabolites, variable methodology/analysis, variable findings/standardization issues, sample destruction, and effects of treatments/ surgery/processing.
Metabolomics future
“We hope to apply the findings of these changes in metabolites to clinical application,” Vallabh concluded. “This is being helped by the development of metabolites databases to register these changes. The hope…is that these metabolite changes may provide novel therapeutic targets.”

Neeru Vallabh PhD
E: neeru.amrita.vallabh@liverpool.ac.uk
Vallabh is a glaucoma specialist at the Liverpool University Hospital NHS Foundation trust and a clinical senior lecturer at the University of Liverpool. She works as a committee member for the UK Eye Genetics group, a section editor for the Eye journal and is a next generation partner in the European Glaucoma Society.
References:
Zhang Q, Wang N, Rui Y, Xia Y, Xiong S, Xia X. New insight of metabolomics in ocular diseases in the context of 3P medicine. EPMA J. 2023;14(1):53-71. doi:10.1007/s13167-023-00313-9
Metabolomics. Metabolomic Technologies Inc. https://www.mtidx.com/our-technology/metabolomics
Steuer AE, Brockbals L, Kraemer T. Metabolomic strategies in biomarker research—new approach for indirect identification of drug consumption and sample manipulation in clinical and forensic toxicology? Front Chem. 2019;7;319. doi:10.3389/fchem.2019.00319
Mass spectrometry. Priyam Study Centre Learning Chemistry. https://www.priyamstudycentre.com/2022/02/mass-spectrometry.html
Jones A. Teaching animations. Warwick School of Life Sciences. Updated March 12, 2024. https://warwick.ac.uk/fac/sci/lifesci/research/sigtraf/animations/
Onissiphorou K. A level chemistry revision: organic chemistry – nuclear magnetic resonance spectroscopy. ReAgent. May 6, 2022. https://www.chemicals.co.uk/blog/a-level-chemistry-revision-organic-chemistry-nuclear-magnetic-resonance-spectroscopy
https://microbiologynote.com/infrared-ir-spectroscopy-principle-instrumentation-application/
Contreras AV, Cocom-Chan B, Hernandez-Montes G, Portillo-Bobadilla T, Resendis-Antonio O. Host-microbiome interaction and cancer: potential application in precision medicine. Front Physiol. 2016;7:606. doi:10.3389/fphys.2016.00606
Wang Y, Hou XW, Liang G, Pan CW. Metabolomics in glaucoma: a systematic review. Invest Ophthalmol Vis Sci. 2021;62(6):9. doi:10.1167/iovs.62.6.9
Tang Y, Shah S, Cho KS, Sun X, Chen DF. Metabolomics in primary open angle glaucoma: a systematic review and meta-analysis. Front Neurosci. 2022;16:835736. doi:10.3389/fnins.2022.835736
Tribble JR, Otmani A, Sun S, et al. Nicotinamide provides neuroprotection in glaucoma by protecting against mitochondrial and metabolic dysfunction. Redox Biol.2021;43:101988. doi:10.1016/j.redox.2021.101988
Zeleznik OA, Kang JH, Lasky-Su, J. et al; UK Biobank Eye and Vision Consortium. Plasma metabolite profile for primary open-angle glaucoma in three US cohorts and the UK Biobank. Nat Commun. 2023;14(1):2860. doi:10.1038/s41467-023-38466-w
Sentosa R, Stiebing C, Eibl M, et al. Targeting the molecular information of the retina with Raman spectroscopy. Invest Ophthalmol Vis Sci. 2023;64(9):PP0023.
Cakir B, Tomita Y, Yagi H, et al. In vivo noninvasive mitochondrial redox assessment of the optic nerve head to predict disease. PNAS Nexus. 2023;2(5):pgad148. doi:10.1093/pnasnexus/pgad148

Newsletter
Don’t miss out—get Ophthalmology Times updates on the latest clinical advancements and expert interviews, straight to your inbox.




