Quest for data increases debate for anti-VEGF therapy approach
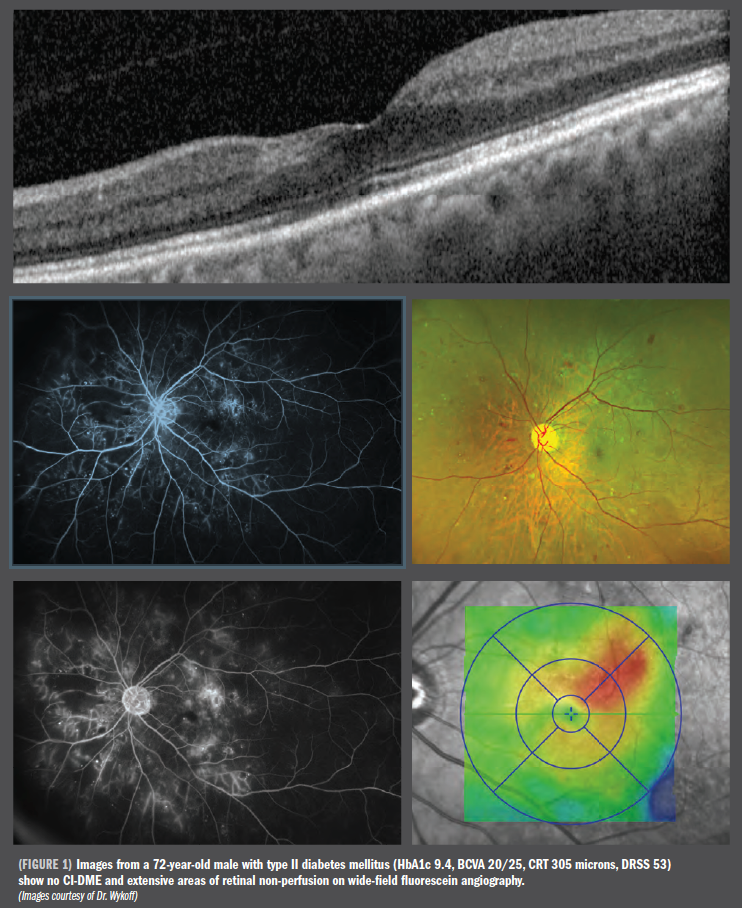
The threshold for treatment with an anti-VEGF agent for diabetic retinopathy wihtout diabetic macular edema is controversial. Two Phase III studies are now investigating aflibercept for moderately severe to severe nonproliferative diabetic retinopathy.
This article was reviewed by Dr. Charles C. Wykoff, MD, PhD
The threshold for initiating anti-VEGF therapy for an eye with diabetic retinopathy (DR) without diabetic macular edema (DME) is currently a matter of debate, and reasonable arguments can be made both in favour and against treating high-risk eyes with moderately severe to severe high-risk nonproliferative disease (NPDR).
More insight on this issue is expected to be forthcoming as data accumulate from prospective studies and are used to develop prognostic deep-learning algorithms.
Related: Anti-VEGF expansion treats all DR stages
In addition to considering interventional management of eyes with NPDR on an individualised basis, ophthalmologists would do well to concentrate on strategies that will bring more patients with diabetes in for ophthalmic care, said Charles C. Wykoff, MD, PhD, at the 2019 meeting of the American Academy of Ophthalmology.
“I do believe there is a signal that some patients with high-risk NPDR benefit from earlier intervention with anti-VEGF therapy,” said Dr Wykoff, director of research, Retina Consultants of Houston and the Greater Houston Retina Research Foundation (GHRRF), and deputy chairman of Ophthalmology, Blanton Eye Institute, Houston Methodist Hospital, Houston, TX, USA. “The problem we face is identifying who these patients are.”
Dr Wycoff pointed out that while additional information is gathered, there are additional challenges to be faced.
“I think a greater challenge than whether or not we should treat patients with NPDR without DME is simply to get these patients to undergo the recommended retinal screening exams,” he said. “Unfortunately, a minority of patients with diabetes in the United States today are coming in for screening before they develop visual problems. We can do better.”
Related: AI model predicts future DR progression in individual patients
Existing guidance
The current AAO Preferred Practice Pattern for DR, which was last updated in 2017, states that for severe NPDR, panretinal photocoagulation or intravitreal anti-VEGF therapy can be considered sometimes. Thus, the guidelines suggest individualising therapy decisions rather than providing specific direction on a population basis.
A role for anti-VEGF therapy in the treatment of high-risk NPDR derives from data collected in the DME registration trials for anti-VEGF agents. Data from studies of aflibercept (Eylea, Regeneron) and ranibizumab (Lucentis, Genentech) showed that with regular injections, approximately one-third of eyes with centre-involved DME (CI-DME), vision loss and DR, had a ≥two-step improvement in their Diabetic Retinopathy Severity Scale (DRSS) score.
Stratification showed that eyes with moderate to severe NPDR (DRSS score of 47 to 53) were particularly likely to benefit with the DRSS improvement relative to those with mild disease or PDr.
Because of such data, two large and rigorously designed Phase III trials were initiated to investigate the safety and efficacy of aflibercept treatment for moderately severe to severe NPDR in patients with good baseline vision.
Related: Sustained DRSS improvement with aflibercept
DRCR.net Protocol W, which is fully enrolled with 322 participants that have clinically-diagnosed severe NPDR without DME and VA ≥20/25, is comparing sham treatment and intravitreal aflibercept 2 mg × two monthly injections and then every 16 weeks (Q16W) for 2 years, then treatment as needed for 2 years. Results from the primary endpoint are not anticipated for about 2 years, Dr Wykoff said.
Primary outcome data are available from PANORAMA that randomly assigned 402 patients with moderately severe to severe NPDR without DME and VA ≥20/40 to sham injection, aflibercept 2 mg Q16W (following four loading doses), or aflibercept 2 mg Q8W (following five loading doses).
Baseline data showed that enrolled patients had excellent vision (mean visula acuity 20/25) with normal central retinal thickness in these eyes without DME. Most eyes were asymptomatic
The primary endpoint analysed the proportion of eyes with a ≥two-step improvement in DRSS at weeks 24 and 52, and the results showed a highly statistically significant difference favouring both the aflibercept Q16W and Q8W groups versus sham treatment at 52 weeks. (65% and 80% versus 15%, respectively).
Related: Anti-VEGFs pose no additional risk of SAEs over other therapies
A secondary endpoint analysis showed vision was generally stable in all groups. At 1 year, the aflibercept groups had a mean gain of approximately 1.5 letters and patients in the sham injection arm gained an average of 0.3 letters.
According to Dr Wykoff, the patients in PANORAMA started with excellent vision, and so there was no reason to expect that they would have gained vision.
“For the most part, the primary issue for these patients is that they are able to see well and maintain their vision,” he said. “What their fundus photographs look like is of secondary concern to most patients.”
Considering such clinically relevant endpoints, Dr Wykoff suggested that the most interesting finding from the 1-year data collected in PANORAMA comes from the prespecified safety analysis looking at the development of PDR and CI-DME, clinical endpoints that physicians and patients would prefer to avoid.
Related: Meta-analysis on DME treatments updated to include treatment regimen
This meaningful secondary analysis showed the rate of progression to PDR or CI-DME, was significantly lower in the aflibercept Q16W and Q8W arms compared with sham (11% and 10% versus 41%, respectively).
“The benefit of aflibercept Q8W and Q16W represents a relative reduction in each of these categories of about 70–85%,” Dr Wykoff said.
Dr Wykoff pointed out that researchers know they can take eyes that have many haemorrhages and turn them into eyes with fewer haemorrhages.
“The question of whether that affects long-term outcome and/or
treatment burden is still being studied, and sets up a theoretical debate between those who believe this data is paradigm shifting and those who think it is important data but at this time does not change practice pattern,” he said.
Pros versus cons
Dr. Wykoff identified several arguments favoring both sides of the debate.
The idea that anti-VEGF treatment is paradigm shifting is supported by the data that shows earlier treatment significantly decreases the probability of developing PDR or CI-DME, both of which can be used as indications to initiate treatment.
Second, there is evidence from studies of anti-VEGF therapy for CI-DME with vision loss and for exudative age-related macular degeneration that earlier treatment initiation is associated with a better long-term outcome and perhaps a reduced treatment burden over the long-term.
Related: Risk factors and recommendations for DR, DME
In addition, and perhaps most interesting according to Dr Wykoff’s perspective, it is known that anti-VEGF treatment can slow the development and progression of retinal nonperfusion, which is believed to be the core vascular pathology underlying DR.
There are population-based data indicating that NPDR without DME is associated with reduced visual function and quality of life.
Recent data from the IRIS registry show that, in the era when anti-VEGF and laser treatment are readily available, severe NPDR in addition to PDR predict rates of vision loss and blindness that are higher than in eyes with earlier stages of DR.
Related: Anti-VEGFS can improve DRSS in majority of patients
In comparison, those who believe that the data from PANORAMA is interesting but do not support widespread use of anti-VEGF treatments for high-risk NPDR without DME at this time can point to the fact that 59% of sham eyes did not develop PDR or CI-DME. Therefore, routine treatment would result in patients receiving injections that may not be required.
Second, intravitreal injections do carry risks.
Third, there are no data at this time to show that anti-VEGF treatment initiated before an eye develops early PDR or DME improves long-term functional outcomes or reduced treatment burden.
Finally, there is a need for longer-term data on compliance with treatment and durability of the benefit.
Read more retina and anti-VEGF content
Dr. Charles C. Wkyoff, MD, PhDE: ccwmd@houstonretina.com
This article was adapted from Dr. Wycoff's presentation at the 2019 meeting of the American Academy of Ophthalmology. Dr. Wykoff is a consultant for and performs research in collaboration with companies that market anti-VEGF therapies.

