
Under the terms of the agreement, Kiora Pharmaceuticals and Théa Open Innovation will develop and commercialize KIO-301 for the treatment of inherited retinal diseases in a deal valued at up to $301 million.

Under the terms of the agreement, Kiora Pharmaceuticals and Théa Open Innovation will develop and commercialize KIO-301 for the treatment of inherited retinal diseases in a deal valued at up to $301 million.

A new study out of Guangzhou, China found that individuals younger than 18 years of age and experiencing high myopia are at a high risk of progressively worsening myopic maculopathy.

The agreement settles Zeiss’s lawsuit against Topcon’s U.S.-based subsidiaries that had been set to start this month in U.S. federal court for the Northern District of California.

In a news release, the FDA noted these are copycat eye drop products that consumers can easily mistake for Bausch + Lomb’s Lumify brand eye drops, an over-the-counter product approved for redness relief.

According to the company, the deals from two global Phase III RVO studies will be presented at Angiogenesis, Exudation, and Degeneration 2024.

A University of Houston professor found that the excitement over the results of LLR as a treatment option for patients with myopia may have come too soon, before its efficacy was confirmed. One company is taking exception with the study.

Wilmer's expansive focus and deep bench are advancing the understanding of artificial intelligence tools and potential ophthalmic applications.

The licensing deal includes upfront, development milestones, and sales royalties, with additional considerations throughout the term of the agreement.

Sanders is affiliated with the Retina Group of Washington (RGW) and PRISM Vision Group. He is the first African American and the first PRISM-affiliated physician to serve as president of the ASRS.
Researchers at Mass Eye and Ear have demonstrated that retinal imaging can help predict a person’s risk of developing ocular, neuropsychiatric, cardiac, metabolic, and pulmonary diseases.

LambdaVision is leveraging the microgravity environment of the space station to develop higher-quality implants than is currently possible on Earth.

ACDN-01 is the first-ever RNA exon editor to enter clinical development and the only clinical-stage therapeutic targeting the genetic cause of Stargardt disease. Ascidian expects to initiate enrollment in Phase 1/2 STELLAR study in the first half of 2024.
Ophthalmologist shares pearls from recent roundtable discussion on topic.

David Hutton of Ophthalmology Times talks with Jeffrey Cleland, PhD, President and CEO of Ashvattha Therapeutics, about the company's D-4517.2, a unique nanomedicine technology for the treatment of wet AMD and DME.
While the potential for fundus images is recognized, the methodology and clinical application must be improved.


According to researchers at Johns Hopkins Children’s Center, AI-driven cameras take images of the back of the eye and require no eye drops can be used to close care gaps.
According to the company, favorable safety and tolerability profiles were observed with the first 2 SPVN06 doses across 6 patients. The exploration of SPVN06 in geographic geographic atrophy is set to begin in 2024.

New technology may shift the diagnostic paradigm

A recent study found that despite worldwide reports of ocular inflammatory events being reported after patients received COVID-19 vaccinations, a low amount has been reported in the UK.

Physicians note the impact of prism lenses can be life-changing for patients.

Genetic testing is proving to be an evolving technology for ophthalmologists.


According to the company, the research is being conducted in collaboration with London-based Imperial College Healthcare NHS Trust.
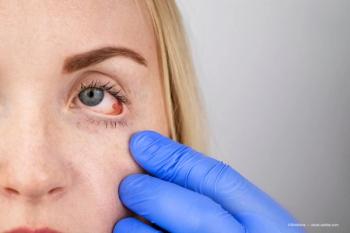
Authors believe that PVI and PHMB are not associated with postoperative ocular irritation.

Evaluating injections of anti-VEGF drugs into the intra-anterior chamber may be a viable alternative, but further study is required.

The new contact lenses contain micro-sensors which monitor changes in IOP over a period of several hours, sending the data collected wirelessly so it can be analyzed by an ophthalmologist and a diagnosis given.

The recall is “due to potential safety concerns after FDA investigators found insanitary conditions.”

Two retina specialists participated in an Ophthalmology Times case-based roundtable discussion and shared their experiences using the anti-VEGF biosimilars, ranibizumab-eqrn and ranibizumab-nuna to manage retinal diseases.