
According to the company, the data demonstrates sustained safety and efficacy in the treatment of the signs and symptoms in patients diagnosed with dry eye disease.

According to the company, the data demonstrates sustained safety and efficacy in the treatment of the signs and symptoms in patients diagnosed with dry eye disease.
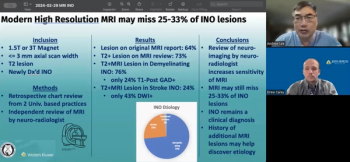
Andrew Lee, MD, and Andrew Carey, MD, sit down on another episode of the NeuroOp Guru to discuss how modern high-resolution MRIs may miss up to a third of internuclear ophthalmoplegia (INO) lesions
The company noted its OCT reference database now offers a library of 870 healthy eyes, more than tripling the previous versions.

The gel is a cross-linked hyaluronic acid derivative and has been cleared by the FDA to temporarily block tear drainage by the occlusion of the canalicular system.
According to the company, the treatment option can save time in the operating room and make the most of corneal donations.
The electromagnetic driving systems are proposed for the flexible 5-DOF magnetic manipulation of a micro-robot within the posterior eye, enabling precise targeted drug delivery.

The launch of Blink NutriTears follows a clinical trial that demonstrated improved ocular symptom severity and tear film homeostasis, and that it helped tears stay on the eyes for 33% longer

The high-level analysis found that 82% of TEPEZZA-treated patients in the follow-up did not report needing any additional TED treatment for nearly 2 years.

Mark Barakat, MD, sat down with Ophthalmology Times to discuss intraocular pressure outcomes with intravitreal injection of aflibercept, 8mg and 2mg in patients with diabetic macular edema through week 48 of the phase 2/3 PHOTON trial.
Understanding how the intricate networks of blood vessels in the eye and brain are formed ultimately could inspire new treatments for conditions like diabetic retinopathy and stroke.

Sean Adrean, MD, FAAO, sat down with Ophthalmology Times to discuss a post hoc analysis of the phase 2/3 PHOTON trial and the outcomes of patients with DME and baseline BCVA of 20/50 or worse or 20/40 or better who were treated with aflibercept 8 mg and 2 mg.

Ocugen is developing OCU410 as a 1-time gene therapy for the treatment of geographic atrophy. It utilizes an AAV delivery platform for the retinal delivery of the ROR Related Orphan Receptor A gene.

The organization is providing free cataract resources for patients and professionals, including fact sheets, social media graphics, a dedicated web page and expert video.

Deepak Sambhara, MD, sat down with Ophthalmology Times to discuss the impact of baseline central retinal thickness (CRT) on vision among patients with diabetic macular edema (DME), as a post hoc analysis of the phase 2/3 PHOTON trial.

EyeBio is developing a pipeline of clinical and preclinical candidates for the prevention and treatment of vision loss associated with retinal vascular leakage.

According to researchers at Waseda University in Japan, the proposed device can measure electrical potentials from different places in the retina simultaneously, which is useful in diagnosing eye diseases.

The COVID-19 pandemic changed medicine forever, and now scientists are forging a new path for healthcare in the future, including in ophthalmology.

The UME module adds to the list of ophthalmic diseases available in the dataset.
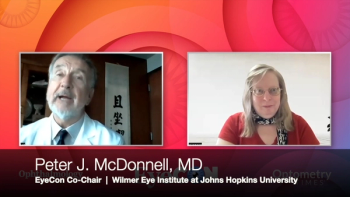
McDonnell stays focused on helping the next generation of ophthalmologists launch their careers in academics.

According to the company, its Phase 3 program enrolled 1,984 patients across COAST and ShORe trials. Topline data from both pivotal trials are expected in 2025.

The FDA recently approved Biologics' Yesafili as well as Samsung Bioepis and Biogen's Opuviz, both close copies of Regeneron Pharmaceutical’s Eylea. The approval offers ophthalmologists and patients additional therapeutic options.

Data from the trial found that MyopiaX proved clinically safe and tolerable, in addition to showing clinical effect on the rate of myopia progression.

The company is in the process of seeking regulatory approvals for the lens in the US and Europe.

Abbreviated New Drug Applications seek potential approval of a generic drug product.

The AIOLIS will be used to clinically evaluate patients’ perception of visual disturbances following premium IOL cataract surgery.
Investigators undertook a secondary analysis of the effects of long-term, low-dose aspirin in over 3,000 older adults who satisfied the inclusion criteria in the Aspirin in Reducing Events in the Elderly–AMD (ASPREE-AMD) study.

All patients included in the study had been referred to Ocular Oncology Service of the Federal University of São Paulo with the suspicion of ocular metastasis.
InflammaDry is a diagnostic test that detects elevated levels of MMP-9, an inflammatory marker consistently found in the tears of patients with dry eye disease.
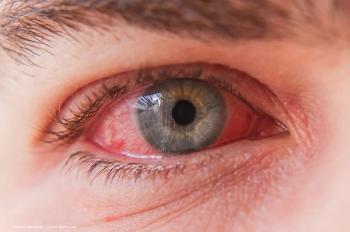
The first case was reported in Texas in a dairy worker in March, while an Australian child with a different strain than the current one in the US was confirmed as well in May.

The hypothesis is based on a post hoc analysis of data from 2 phase 3 clinical trials of pegcetacoplan by Dun Jack Fu, PhD, and colleagues.