
BI 771716, with technology licensed from CDR-Life, is a highly specific antibody fragment, possibly enabling an optimized penetration through all retinal layers to the most critical target site driving GA disease pathology.

BI 771716, with technology licensed from CDR-Life, is a highly specific antibody fragment, possibly enabling an optimized penetration through all retinal layers to the most critical target site driving GA disease pathology.
MK-3000, formally known as EYE103, is an investigational, potentially first-in-class tetravalent, tri-specific antibody that acts as an agonist of the Wnt signaling pathway.
The trial is evaluating phentolamine ophthalmic solution 0.75% for the treatment of presbyopia.

Investigators at the Dean McGee Eye Institute reviewed the accuracy of the tools in measuring 5 vision assessment parameters, including distance visual acuity, near visual acuity, color vision testing, contrast sensitivity, and pupillary distance.

Prevent Blindness is planning a series of events and program partnerships leading up to World Sight Day on Oct. 10, 2024.
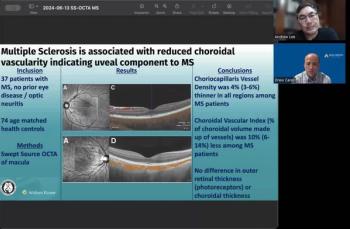
Andrew Lee, MD, and Andrew Carey, MD, sit down on another episode of the NeuroOp Guru to discuss Multiple Sclerosis and its association with reduced choroidal vascularity.

The non-diffractive, aspheric IOLs are the second generation of BVI’s ISOPURE family

The one-time intravitreally delivered gene therapy is for the treatment of retinitis pigmentosa (RP).

A team of researchers at the Center for Regenerative Therapies Dresden of Dresden University of Technology is working to develop a method to replace or regenerate damaged photoreceptor cells in the human retina.

Data from this trial will be reported ahead of AAO 2024 in Chicago, Illinois.

This Week in Ophthalmology is a video series highlighting some of the top articles featured on the Ophthalmology Times website.

The IOL, designed with artificial intelligence, will launch at this year's ESCRS Congress in Barcelona, Spain

A team of researchers from Johns Hopkins Medicine and the University of Wisconsin-Madison conducted a study on the application of autonomous artificial intelligence and testing for diabetic eye disease.

The American Academy of Ophthalmology indicates that consumers who may see the eye color-changing drops advertised and used on TikTok or elsewhere online should be advised that they are not FDA approved.

The day will be held on October 10. It is coordinated by the International Agency for the Prevention of Blindness under the “Love Your Eyes” campaign banner, and is putting children at the center of the campaign.

Australian researchers have found that clinical registries may be an untapped font of information for artificial intelligence.
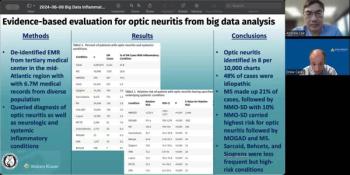
Andrew Lee, MD, and Andrew Carey, MD, sit down on another episode of the NeuroOp Guru to discuss evidence-based evaluation for optic neuritis from big data analysis

The product candidate is a modifier gene therapy for broad retinitis pigmentosa indication.

Researchers at the Medical College of Georgia at Augusta University are teaming up with Polaris Dawn to gain a better understanding of how eye changes many astronauts experience during spaceflight could ultimately leave them with multiple symptoms once they return to Earth.

The drop improves vision by breaking up degraded protein complexes that cause lens clouding

According to researchers at Nanyang Technology University Singapore, smart contact lenses can be used to correct vision, monitor the health of the user, and flag and treat diseases for patients diagnosed with chronic health conditions such as glaucoma and diabetes.

In a 10-year retrospective study, a team of researchers in China found the top 2 categories for cancellations were medical factors and patient-related factors.

This Week in Ophthalmology is a video series highlighting some of the top articles featured on the Ophthalmology Times website.
CN6 palsy from the COVID-19 virus is rare, and can occur in infants as young as 7 months.

The Phase 1 trial is a multicenter, open-label, dose-escalation safety clinical trial, with 18 subjects, who each have received a single periocular injection of AIV007
The option is a definitive step forward in the field of vitreoretinal surgery

The next step for the research likely will focus on determining whether blocking GSK3 can reverse existing AMD damage, potentially leading to new treatment options.

Rationale for delving into this topic is the recent studies that have reported that active and healthy living can reduce IOP.

At the World Ophthalmology Congress in Vancouver, Canada, John D. Sheppard, MD, MMSc, FACS, presented data on perfluorohexyloctane ophthalmic solution in the treatment of dry eye. He sat down with Ophthalmology Times to discuss the topic in further detail.