
A Q&A with PPP Committee Cornea/External Disease Panel member Daniel S. Choi, MD.

Melt Pharmaceuticals announced positive results from its Phase 3 study of MELT-300, a non-IV, non-opioid tablet for procedural sedation during cataract surgery. The results support a regulatory submission, with potential to revolutionize sedation practices in various medical specialties.

A discussion with Shigeru Kinoshita, MD, PhD; and Edward J. Holland, MD.

The EnVision Summit, co-founded by Bonnie An Henderson, MD, is a unique family-oriented, multispecialty ophthalmology CME conference, along with a newly introduced optometry track. Henderson sits down with Ophthalmology Times to talk about the 2025 conference that will take place February 14 to 17 at the Caribe Hilton San Juan, in Puerto Rico.
A PDUFA decision date of April 2, 2025 has been assigned
SpyGlass Pharma completed enrollment in a Phase 1/2 trial evaluating its drug delivery platform for glaucoma and ocular hypertension. Designed for implantation during cataract surgery, the platform delivers bimatoprost to lower intraocular pressure for up to 3 years.

Astellas said in a press release that the FDA comments in the CRL relate to proposed labeling language, not safety.

Key findings include reduced lesion growth, retinal tissue preservation, and improved visual function, demonstrating significant potential for the treatment option.


The designations underscore the unmet need in Stargardt disease, a rare and serious pediatric disease for which no treatment exists.
The biosimilar now known by the brand name OPUVIZ was previously known as the biosimilar candidate SB15.

This retrospective cohort study was designed to provide further information on the association between BP parameters and VF progression over time.

Emmecell announced positive Phase 1 extension study results for EO2002, a nonsurgical cell therapy for corneal edema. The therapy improved vision, reduced central corneal thickness, and demonstrated strong safety, offering an alternative to corneal transplants.

Retreatment rates are lower, with few orbit decompression procedures.

This Week in Ophthalmology is a weekly video series highlighting the latest news and articles featured on the Ophthalmology Times website.

A coalition of eye health advocacy groups vocalized support for the legislation
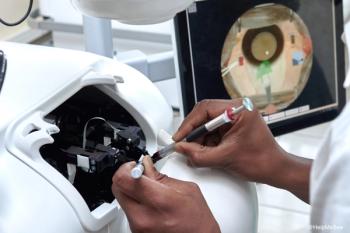
HelpMeSee’s Mazava Project trains general practitioners in Madagascar to perform cataract surgeries, addressing the country’s high rate of avoidable blindness. Trainees gain proficiency through simulation-based learning and live surgeries, improving access to eye care in remote areas.
Livionex, recently announced a successful end-of-Phase 2 meeting with the FDA, supporting the advancement of C-KAD, a 2.6% EDTA ophthalmic solution, to a Phase 3 clinical study. In an interview with Ophthalmology Times, Randall Olson, MD, lead author and a Distinguished Professor at the University of Utah, Chair of the Department of Ophthalmology and Visual Sciences, and CEO of the John A. Moran Eye Center, discusses the ongoing research.

Afqlir (aflibercept) is a 2 mg vial kit and pre-filled syringe for intravitreal injection.
Prevent Blindness has designated November 18 to 24, 2024, as the 5th annual Thyroid Eye Disease (TED) Awareness Week. The week aims to raise awareness of TED's symptoms, impact on vision and mental health, and new treatment options. Educational resources and expert-led episodes will support patients and healthcare professionals.

Sun Pharmaceutical presented Phase 4 data at ESCRS 2024 showing that cyclosporine ophthalmic solution 0.09% significantly improves dry eye disease symptoms and corneal staining in patients inadequately controlled on Restasis, with positive results observed through 12 weeks of treatment.

Program director and co-founder Bonnie An Henderson, MD, sits down with Ophthalmology Times to talk about how the annual conference is a unique blend of cutting-edge science, professional growth, and community. The 2025 conference will take place February 14 to 17 at the Caribe Hilton San Juan, in Puerto Rico.
HelpMeSee has partnered with new initiatives to address the global cataract blindness crisis, focusing on empowering women in low- and middle-income countries. Through training and education, the organization aims to reduce gender inequality and improve socioeconomic outcomes worldwide.

HelpMeSee has partnered with Empowered, an educational series hosted by Meg Ryan, to spotlight the cataract blindness crisis, its socioeconomic impacts, and gender inequality issues. The episode emphasizes simulation-based surgical training advancements, expanding cataract care access for underserved populations and fostering equitable healthcare solutions.

NEI Initiative aims to boost awareness, standardize diagnosis, and improve care for children with CVI through multidisciplinary collaboration and emerging technologies.

Atsena Therapeutics and Nippon Shinyaku have formed an exclusive licensing agreement for ATSN-101, a gene therapy for Leber congenital amaurosis (LCA1). Nippon Shinyaku will commercialize ATSN-101 in the U.S. and Japan, while Atsena retains global rights outside these territories.

Study highlights safety, efficacy of device in managing persistent DED.
A recent cohort study revealed low adoption of FDA-approved AI-based diabetic retinopathy detection, with less than 5% of diabetic patients receiving ophthalmic imaging. Researchers emphasize the need for improved awareness, cost-effectiveness, and integration to increase diabetic retinopathy screening rates.

The Healthcare Private Equity team has advised Retina Consultants of America (RCA) and Webster Equity Partners in the sale of RCA to Cencora.

The weekly drug-eluting contact lens was created by the company’s proprietary 3D printing technology and met all primary endpoints with no serious adverse events reported in either cohort.