
Children experienced sudden vision loss, visual field constriction, nyctalopia and dyschromatopsia following febrile illness.

Children experienced sudden vision loss, visual field constriction, nyctalopia and dyschromatopsia following febrile illness.
Direct selective laser trabeculoplasty enhances the experience for both patients and physicians, he notes, while also mitigating issues related to poor adherence and ocular surface disease from topical therapy.

At the Envision Summit 2025 in San Juan, Puerto Rico, Kelly Donovan, MD, talked about her graduate research on the earliest phase of diabetic retinopathy, and trying to understand how hyperglycemia translates into disease.

Sexual harassment in the specialty remains high 5 years after a previous study, with higher frequencies of recent experiences and continued low reporting rates.

At the Envision Summit 2025 in San Juan, Puerto Rico, Oleg Alekseev, MD, PhD, talked about difficulties in diagnosing inherited retinal degenerations due to mutations in over 300 different genes.
The UK-based company will debut the tool, called Dr.Noon CVD, at two conferences in March.

At the Envision Summit 2025 in San Juan, Puerto Rico, Ollya Fromal, MD, gave advice on how retina specialists can manage add-ons in their busy retina clinics.

At the Envision Summit 2025 in San Juan, Puerto Rico, Giulia Corradetti, MD discussed AI applications in the identification and prediction of OCT structural biomarkers in intermediate AMD.

Ying Han, MD, PhD discusses glaucoma management strategies, including transitioning care between ophthalmologists and optometrists, improving diagnosis, and the hope for future glaucoma developments.

Patients who underwent bilateral treatment experienced a higher rate of clinically relevant recovery, the authors reported.
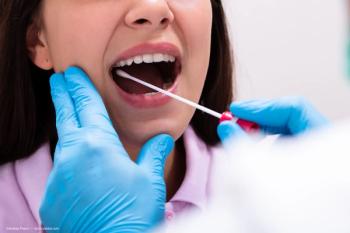
SightScore is a saliva test that looks at millions of genetic variants in the patient’s genome to create a personalized polygenic risk score for glaucoma.

At the Envision Summit 2025 in San Juan, Puerto Rico, Sharon Fekrat, MD, FACS, FASRS talked about multi-modal retinal and choroidal imaging to diagnose and identify neurodegenerative diseases using machine learning.

The focus in recent years has shifted to reducing the long-term burden of medical therapy, minimizing the risk of medication toxicity and persistent ocular surface disease.

Shah discusses several studies from the IRIS registry and plans for a retina program at next year's event to address the growing interest in retina procedures and techniques.

The new first-line laser treatment for glaucoma and ocular hypertension will be unveiled at the 2025 American Glaucoma Society annual meeting in Washington, DC, from February 26 to March 2.

At the Envision Summit 2025 in San Juan, Puerto Rico, Rachel Simpson, MD, talked about new developments in glaucoma treatment and really how to tailor glaucoma treatment to the patient.
MonacoPro, the next evolution of Monaco from Optos, retains the powerful ultra-widefield SLO and spectral domain imaging while adding additional key product features.

At the Envision Summit 2025 in San Juan, Puerto Rico, Angela Zhu, MD, offered advice on cataract surgery for all ages, focusing on pediatrics.
Advancements in drug durability and patient care are closing treatment gaps.
AVT06 is Alvotech’s proposed biosimilar to Eylea (aflibercept) 2mg.

At the Envision Summit 2025 in San Juan, Puerto Rico, Sherrol Reynolds, OD, FAAO, participated in a discussion on patient-centered care and the newest treatment strategies in neovascular AMD and DME.

At the Envision Summit 2025 in San Juan, Puerto Rico, Ashley Wallace Tucker, OD, FAAO, FSLS, gave insight into advancements in keratoconus management and the importance of early detection as well as tips and advice on fitting scleral contact lens
Examining the performance of AI algorithms versus human graders in neovascular age-related macular degeneration

At the Envision Summit 2025 in San Juan, Puerto Rico, Lana Rifkin, MD, gave clinicians advice on how, and when it may be most appropriate to use the new G2211 code.

At the Envision Summit 2025 in San Juan, Puerto Rico, Arjan Hura, MD, talked about EVO ICL surgery, its benefits, and how every ophthalmologist has the ability and training to conduct the surgery even if they aren't aware.

At the Envision Summit 2025 in San Juan, Puerto Rico, Nita Valikodath, MD, MS, gave insight into a challenging case of a 14-year-old boy who had trauma-related retinal detachment in his right eye, where a further macula-on retinal detachment with retinal dialysis was found in the left.

At the Envision Summit 2025 in San Juan, Puerto Rico, Deepak Sambhara, MD, gave insight into the 96-week post hoc fluid outcomes analysis of patients who participated in the phase 3 clinical trial PULSAR.

At the Envision Summit 2025 in San Juan, Puerto Rico, Carol Karp, MD, discussed new research presented by her fellows at the meeting on AI for the diagnosis of ocular surface tumors as well as a new "tear biopsy" for the detection of cancer.

At the Envision Summit 2025 in San Juan, Puerto Rico, Brittany Simmons, MD, discussed periorbital injuries and how they can affect the soft tissue and bones around the eye and gives insight into how clinicians should best go about treating it.

At the Envision Summit 2025 in San Juan, Puerto Rico, Jessica Steen, OD, FAAO talked with glaucoma surgeons on the importance of comanaging patients throughout their lifetime.