
KAIST researchers unveil a new microbial strain for efficient, eco-friendly lutein production.

KAIST researchers unveil a new microbial strain for efficient, eco-friendly lutein production.

MCO-010 is a gene-agnostic therapy for retinitis pigmentosa.

KALA Bio completes enrollment in CHASE trial, advancing KPI-012 for treating persistent corneal epithelial defects. Topline results are expected by the end of Q3 2025.


Preliminary results of PST-611-CT1 are anticipated early 2026, subject to patient recruitment.

MHRA issues precautionary recall for Zaditen eye drops due to potential microbial contamination risk

Boehringer Ingelheim initiates the THULITE study, exploring an oral treatment for diabetic macular edema.
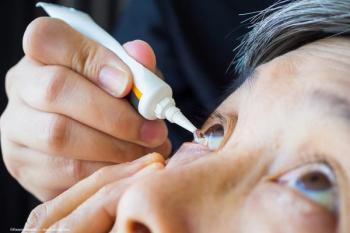
Lupin Limited secures FDA approval for Loteprednol Etabonate Ophthalmic Gel, enhancing treatment options for postoperative ocular inflammation and pain.

The VAC will be a strategic team informing Nanoscope’s scientific, clinical, and community-facing efforts.

PYC Therapeutics advances VP-001 for retinitis pigmentosa, gaining FDA insights for its upcoming registrational trial.

New findings reveal RG6501 cell therapy shows promising long-term visual improvements for geographic atrophy patients

The novel preservative-free eye drop therapy for glaucoma aims to improve IOP control and patient adherence with a fixed-dose combination.


Centennial Optical distributes ophthalmic frames, eyeglass lenses, sunglasses, lab supplies, and optical accessories.

Prevent Blindness offers resources for Cataract Month, empowering patients and caregivers with knowledge on cataracts and surgery options.

Telomir-1 shows promising results in restoring vision and retinal structure in age-related macular degeneration, marking a significant advancement in treatment.

Dilraj S. Grewal, MD, FASRS, provides an in-depth overview of the current state of research and treatment innovations.

Vakharia insights from the ARCHER trial on geographic atrophy treatment for dry AMD, highlighting promising results and future directions in ophthalmology.

This partnership aims to address care gaps by identifying untreated and undertreated patients at scale.

Sunir Garg, MD, discusses a challenge in ophthalmological procedures: patient discomfort during intravitreal injections caused by povidone iodine.
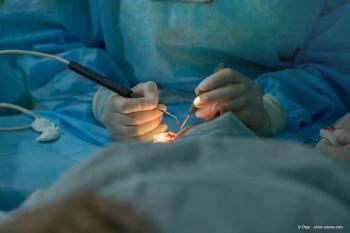
The new surgical technique incorporates a new surgical clamp that maintains eye pressure during the insertion of 2 tissue patches in immediate succession while minimizing damage to the surrounding tissue.

The designation is based off previously reported phase 3 data from the ongoing DRAGON trial.
Ashvattha Therapeutics reveals promising phase 2 results for migaldendranib, showcasing significant improvements in retinal disease treatment and reduced injection burden.

SYL1801 is an investigational siRNA therapy administered via eye drops, rather than intravitreal injection, to treat nAMD.
New research reveals how low blood sugar can damage the retina in diabetes, highlighting potential treatments targeting specific proteins to prevent vision loss.
Optigo Biotherapeutics presents promising preclinical data at ARVO.

The VAN-2401 phase 1 clinical trial will evaluate the use of KH658 for the treatment of wet AMD.
