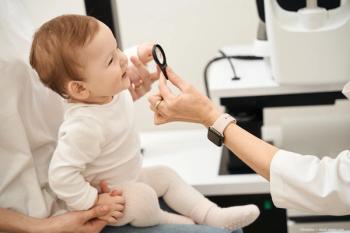
Bioptigen to study SD-OCT use in infants
Medical device company Bioptigen Inc. will use a $2.7 million, 27-month grant from the National Eye Institute to study the use of portable spectral-domain optical coherence tomography (SD-OCT) systems in researching, diagnosing, and treating eye diseases in premature and neonatal infants..
Research Triangle Park, NC-Medical device company Bioptigen Inc. will use a $2.7 million, 27-month grant from the National Eye Institute to study the use of portable spectral-domain optical coherence tomography (SD-OCT) systems in researching, diagnosing, and treating eye diseases in premature and neonatal infants.
“Children have unique eye-care needs,” said Eric L. Buckland, PhD, president and chief executive officer of Bioptigen. “It is both exciting and satisfying to work on imaging tailored to the developing eye. Our objective is to provide researchers and clinicians with the comprehensive, mobile imaging they require to view and manage pediatric eye disease properly.”
The company’s SD-OCT device (Envisu) uses low-power incoherent light to generate high-resolution, depth-resolved images of ocular tissues. It acquires and displays volumetric images suitable for real-time exploration of tissue physiology and pathology, according to the company.
In other news, the company has been named a finalist in three categories of the 2011 North Carolina Technology Association (NCTA) 21 Awards. The annual showcase, designed to promote and celebrate innovation and high-tech excellence throughout the state, honors 21 companies and individuals.
The NCTA named Bioptigen a finalist in the Most Innovative Technology Company, Private Company Excellence, and Small Technology Stage of Development categories. Winners will be announced Nov. 10.
“To be named an NCTA 21 Awards finalist alongside the finest of North Carolina’s growing and established firms is exciting for everyone at Bioptigen,” Dr. Buckland said. “I am proud of our employees and the passion they pour every day into developing revolutionary technologies that offer the potential to change our understanding of eye disease and treatment.”
For more articles in this issue of Ophthalmology Times eReport,
Newsletter
Don’t miss out—get Ophthalmology Times updates on the latest clinical advancements and expert interviews, straight to your inbox.