- Ophthalmology Times: April 15, 2020
- Volume 45
- Issue 7
Addressing MGD with thermal pulsation system
The use of a thermal lid pulsation device can prove to be an important component of routine practice for the treatment of the signs and symptoms of meibomian gland dysfunction.
The use of a thermal pulsation device can prove to be an important component of routine practice for the treatment of the signs and symptoms of meibomian gland dysfunction.
Special to Ophthalmology Times®
Affecting more than 30 million Americans,1
Multiple factors contribute to the increasing prevalence of
In obstructive MGD patients, the meibomian glands located in the upper and lower eyelids are blocked.3 Reduced meibum secretion may result in changes to the composition of the tear film, leading to quicker tear evaporation, eye irritation, inflammation, and
Recently, the American Society of Cataract and Refractive Surgery (ASCRS) published an algorithm for the preoperative diagnosis and treatment of OSD, including dry eye.4 This algorithm suggests screening for
Addressing
By improving ocular surface health, symptomatic patients (and those who did not realize they were symptomatic in the first place) may notice less burning and/or irritation. Patients that suffer from symptoms or show signs of
Related: Lab tear tests aid reimbursement, application of dry eye
It is estimated that up to 34% of patients at a routine eyecare clinic may have
Experience at Metrolina Eye Associates suggests that up to 80% of patients at a
Clinicians today have an array of tools at their disposal to optimize pre-operative measurements for both
Despite these technological advancements, the value of any tests conducted pre-operatively depends on having a healthy ocular surface, especially because dry eye causes fluctuating vision.9
With the recent, and uptake of AT-IOLs, accurate refractive readings going into
Related: Personalizing cataract surgery
Managing MGD
In treating dry eye, the underlying disease mechanism must be considered; the eyelids are often identified as a cause of
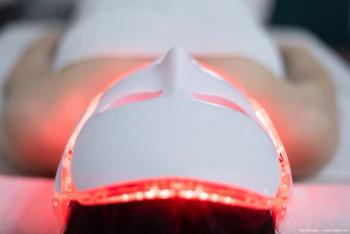
The current approach to addressing MGD at Metrolina Eye Associates involves thermal lid pulsation with a thermal pulsation system (iLUX, Alcon).
The Alcon system is cleared by the FDA10 with clinically demonstrated results in reducing the signs and symptoms of MGD.11 The hallmarks of the system include its portability, rapid treatment for patients, and significant improvements in MGD symptoms.10Related:
Typically, after an initial iLUX treatment, patients will experience objective improvements in Meibomian Gland Score, tear break-up time, and ocular surface disease index.10
Taking the time to address MGD, in general routine eye care as well as prior to refractive or
Patients who are satisfied with their pre-operative care are likely to bring repeat business and refer their family and friends.
If a patient exhibits signs of MGD, it is important to take the time to explain the disease to them (including its chronic, progressive, and irreversible nature), outline their treatment options, and highlight that the thermal pulsation system addresses the root cause of MGD (i.e., clears blocked meibomian glands) and supports a holistic approach to reducing the signs and symptoms associated with MGD.
Even though this conversation only requires two minutes of time, it garners trust from patients and helps to set reasonable expectations.
At Metrolina Eye Associates, the vast majority of patients who have been treated with the thermal pulsation system have experienced a reduction in the signs and symptoms of MGD.
Related:
The thermal pulsation system was first implemented at Metrolina Eye Associates’ clinics approximately 1.5 years ago. Since that time, each clinic has been outfitted with one system and these devices have been used to treat thousands of patients with MGD.
When adopting the thermal pulsation system as part of standard MGD management practice, only minor hurdles were encountered, including ensuring the device was always charged and that enough sterile tips were consistently on-hand. Treatments take less than 10 minutes
There is a spectrum of eye care professionals whose patients may exhibit the signs and symptoms of MGD and could benefit from treatment with the thermal pulsation system.
I urge other eye care professionals to take the time and initiative to identify MGD and consider adopting the use of a thermal lid pulsation device as an important component of routine practice.
Addressing MGD is important not only to reduce the signs and symptoms of MGD, but also to optimize cataract surgery pre-operative measurements, patient comfort, and overall ocular health.4
IMPORTANT PRODUCT INFORMATION
Indication: The iLUX® Device is indicated for the application of localized heat and pressure therapy in adult patients with chronic disease of the eyelids, including Meibomian Gland Dysfunction (MGD), also known as evaporative dry eye.
Contraindications:
Do NOT use the iLUX® Device in patients with the following conditions: Patients whose pupils have been pharmaceutically dilated; patients who have undergone ocular surgery within prior 12 months; patients with ocular injury or trauma, chemical burns, or limbal stem cell deficiency (within prior 3 months); patients with active ocular herpes zoster or simplex of eye or eyelid or a history of these within prior 3 months; patients with cicatricial lid margin disease; patients with active ocular infection, active ocular inflammation or history of chronic, recurrent ocular inflammation within prior 3 months; patients with an ocular surface abnormality that may compromise corneal integrity; patients with lid surface abnormalities that affect lid function in either eye; patients with aphakia; or patients with permanent makeup or tattoos on their eyelids.
Warnings/Precautions:
Federal law restricts this device to sale by or on the order of a licensed healthcare practitioner.
The Disposable may not fit all eyes, such as eyes with small palpebral fornices. Use of the iLUX® device is NOT recommended in patients with the following conditions: moderate to severe allergic, vernal or giant papillary conjunctivitis; severe eyelid inflammation; systemic disease conditions that cause dry eye; in patients who are taking medications known to cause dryness; or patients with punctal plugs.
Potential Adverse Reactions:
Potential adverse effects may include eyelid/eye pain requiring discontinuation of the treatment procedure, eyelid irritation or inflammation, temporary reddening of the skin, ocular surface irritation or inflammation (e.g., corneal abrasion, conjunctive edema or conjunctival injection (hyperemia)), and ocular symptoms (e.g., burning, stinging, tearing, itching, discharge, redness, foreign body sensation, visual disturbance, sensitivity to light).
Attention: Please refer to the User Manual for a complete list of contraindications, instructions for use, warnings and precautions for the iLUX® Device.
Ivan Mac, MD, MBA
E: [email protected]
Dr. Mac is a consultant and/or speaker for Alcon, Allergan, Kala Therapeutics, NovaBay, Sun Pharmaceuticals; and an investor with Ellex, Tear Film Innovations/TearClear/Equinox/Corneagen/RxSight (investor), and Visionary Venture Group.
References:
Tear Film & Ocular Surface Society. TFOS DEWS II Report.
Lemp MA, Crews LA, Bron AJ, Foulks GN, Sullivan BD (2012) Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 31 (5): 472-478.
Baudouin C, Messmer EM, Aragona P, Geerling G, Akova YA et al. (2016) Revisiting the vicious circle of dry eye disease: a focus on the pathophysiology of meibomian gland dysfunction. Br J Ophthalmol. 100 (3): 300-306.
Starr CE, Gupta PK, Farid M, Beckman KA, Chan CC et al. (2019) An algorithm for the preoperative diagnosis and treatment of ocular surface disorders. J Cataract Refract Surg 45 (5): 669-684.
Trattler WB, Majmudar PA, Donnenfeld ED, McDonald MB, Stonecipher KG et al. (2017) The Prospective Health Assessment of Cataract Patients’ Ocular Surface (PHACO) study: the effect of dry eye. Clin Ophthalmol 11 1423-1430.
[No authors listed] (2007) The epidemiology of dry eye diease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop. Ocular Surface 5 (2): 93-107.
Lin PY, Tsai SY, Cheng CY, Liu JH, Chou P et al. (2003) Prevalence of dry eye among an elderly Chinese population in Taiwan: the Shihpai Eye Study. Ophthalmology 110 (6): 1096-1101.
Gupta PK, Drinkwater OJ, VanDusen KW, Brissette AR, Starr CE (2018) Prevalence of ocular surface dysfunction in patients presenting for cataract surgery evaluation. J Cataract Refract Surg 44 (9): 1090-1096.
Clayton JA (2018) Dry Eye. N Engl J Med 379 (11): e19.
FDA US (2016). iLux Premarket Approval Notification. Available online at:
U.S. National Library of Medicine (Web Page) Comparison Between iLux and LipiFlow in the Treatment of Meibomian Gland Dysfunction. Updated May 30, 2019. Available online at:
Chhadva P, Goldhardt R, Galor A (2017) Meibomian Gland Disease: The Role of Gland Dysfunction in Dry Eye Disease. Ophthalmology 124 (11S): S20-S26.
Articles in this issue
almost 6 years ago
PACK-CXL researchers continue to clear hurdles of topical drugsNewsletter
Don’t miss out—get Ophthalmology Times updates on the latest clinical advancements and expert interviews, straight to your inbox.