Rethinking ‘Rundle’s Curve’: How our Understanding of Thyroid Eye Disease (TED) Has Evolved
Our understanding of Thyroid Eye Disease (TED) has changed considerably over the last few years, driven primarily by new treatment options and research. This evolved understanding has prompted specialists in the field to reconsider the course of the disease and how to approach treatment.
Historically, TED has been considered a biphasic disease, using Rundle’s curve to show that the disease changes over time and then enters a stable phase.1 In this conventional time-based model, patients start with “active” or “acute” TED, where inflammatory signs and symptoms such as eye pain, swelling, proptosis and diplopia progress and may worsen. After a few years, the disease is traditionally thought to enter an “inactive” or “chronic” phase where signs, symptoms, and patient burden have been thought to be diminished.2
Current thinking is that TED, in many patients, is a lifelong, heterogeneous autoimmune condition that waxes and wanes and can flare, with indistinct phases. The weight of TED symptoms to patients may change or reduce over time, but they may not completely disappear or become less problematic for those living with the disease.3 It was noted in one study that the quality of life of patients who endured TED for several years was reported to be significantly impaired and nearly identical to scores seen in patients more recently diagnosed with TED. This suggests that patients’ lives can be greatly impacted by TED regardless of disease duration or clinical activity.4 In addition, inflammatory symptoms may return at any time, oftentimes driven by external stressors such as pregnancy, smoking, ocular trauma and other co-morbidities that may not even be thyroid related.5
Evidence now suggests that insulin-like growth factor-1 (IGF-1) is a key driver of the disease, and its related pathways remain upregulated throughout the duration of TED.6 The activation of this disease mechanism, even in patients with longer disease duration or low clinical activation, may help explain why patients with TED of long duration may still struggle with symptoms affecting their daily activities, oftentimes leading to psychological disturbances such as depression and anxiety.7
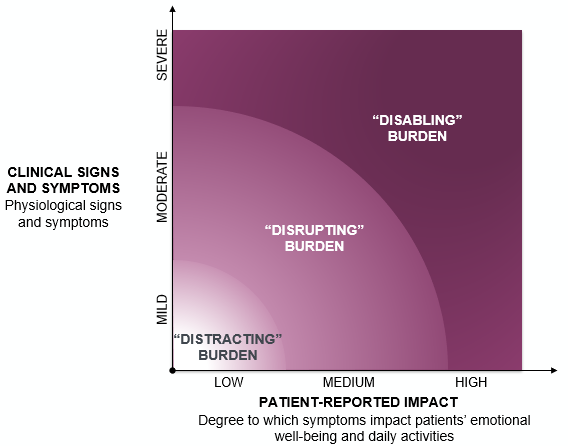
With a newer understanding of disease processes that transcends time, we must strongly consider a symptoms-based approach to treatment and document the burden of disease on our patients’ quality of life (see figure below). This means purposefully asking our patients how symptoms affect them not only physically, but also in their everyday activity and emotional well-being.8 For instance, have they made any modifications to their lifestyle as a result of the disease or noticed changes in their mood or social activities? I have found that my patients are often reluctant to bring up these challenges unless prompted by their physician and may have lost hope on finding any relief for the frustrating symptoms they struggle with. Because symptoms can progress over time, consistent disease burden assessments should be built into every visit.
As physicians caring for TED patients, it’s an exciting time. Research on TED continues to change the treatment paradigm. It is time to accept TED as a heterogenous disease and base our decisions on the true burden of the condition on patients’ lives, not just how long a patient has been dealing with it.
Sponsored by Horizon Therapeutics.
Dr. Kikkawa is the Trude Kahn Hollander Chair in Ophthalmology, Distinguished Professor of Ophthalmology and Plastic Surgery, Chief of the Division of Oculofacial Plastic and Reconstructive Surgery, and Vice Chairman at the University of California at San Diego (UCSD) Department of Ophthalmology and UCSD Shiley Eye Institute in La Jolla. He did not receive financial compensation for his participation in this article.
©2023 Horizon Therapeutics plc DA-UNBR-US-02000 08/23
References
- Barrio-Barrio J, et al. Graves' Ophthalmopathy: VISA versus EUGOGO Classification, Assessment, and Management. J Ophthalmol. 2015;2015:249125.
- Bartley GB. Rundle and His Curve. Arch Ophthalmol. 2011;129(3)356-358.
- Patel P, et al. Recurrent Thyroid Eye Disease. Ophthalmic Plast Reconstr Surg. 2015;31(6):445-8.
- Cockerham K, et al. Quality of Life in Patients with Chronic Thyroid Eye Disease in the United States. Ophthalmol Ther. 2021;10(4):975-987.
- Wang Y, et al. Inflammatory and Noninflammatory Thyroid Eye Disease: Comparison of Disease Signs, Symptoms, and Quality of Life in Patients in the United States. Endocr Pract. 2022. 28(9):842-846.
- Ugradar S, et al. Whole genome transcriptome comparison of acute and chronic thyroid eye disease: emergence of a molecular signature. Presented at: American Academy of Ophthalmology; September 30-October 3, 2022.
- Lee TC, et al. Evaluation of Depression and Anxiety in a Diverse Population With Thyroid Eye Disease Using the Nationwide NIH All of Us Database. Ophthalmic Plast Reconstr Surg. 2023;01;39(3):281-287.
- Farid M, et al. Psychological disturbance in graves ophthalmopathy. Arch Ophthalmol. 2005;123(4):491-6.