Article
KIO-301, an investigational treatment for RP, demonstrates visual function restoration in patients who are blind
Author(s):
In a presentation at the Association for Research in Vision and Ophthalmology annual meeting in New Orleans, Kiora Pharmaceuticals showed its investigational drug has the potential to restore light perception in patients who are blind or living with ultra-low vision due to retinitis pigmentosa.
Intravitreal injection is used to deliver KOI-301. (Images courtesy of Kiora Pharmaceuticals)
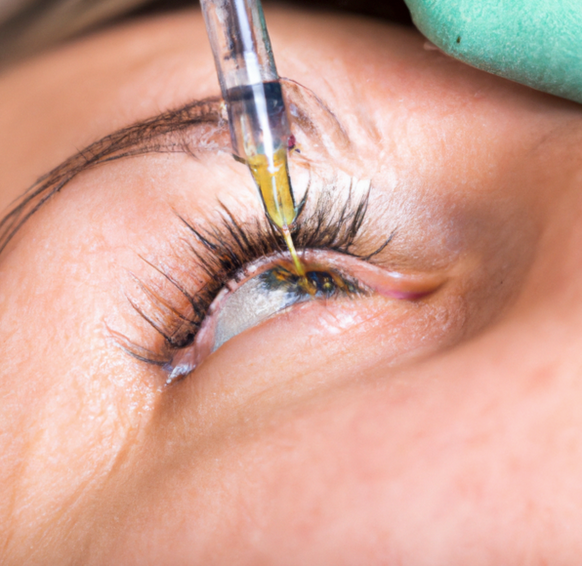
Kiora Pharmaceuticals presented preliminary results from an ongoing clinical trial showing its investigational drug, KIO-301, has the potential to restore light perception in patients who are blind or living with ultra-low vision due to retinitis pigmentosa.
According to the company, KIO-301 is a light-sensing small molecule designed to reactivate visual function of the eye in response to light. The results were presented today at the Association for Research in Vision and Ophthalmology (ARVO) annual meeting in New Orleans.
"These preliminary results suggest that the initial low dose of KIO-301 is capable of improving light perception in patients who have ultra-low vision or are completely blind," Robert Casson, MBBS, M.Biostat, DPhil, FRANZCO, principal investigator of the study from the Royal Adelaide Hospital. "We saw a marked improvement in functional measures, which correlate to neural imaging results that demonstrate increased visual cortex activity in the brain from baseline to two weeks after treatment."
The company noted quantitative and qualitative measures from early patients in the study were reported. The highlighted case study presented is a patient with no light perception and received a low dose injection of KIO-301 in one eye.
The company noted the following observations were reported:
- Patient-reported improvement in the ability to perceive a contrast between light and dark at days seven 14 and 29.
- Improvement in object identification.
- Positive impact in overall functional vision as it relates to the use of vision in everyday activities.
- Substantial improvement in quality of life, as measured by the National Eye Institute's visual functional questionnaire. There was an increase of six points (an increase of two to four points is considered clinically meaningful1).
- Imaging data, using functional MRI, showed significantly increased activity in areas of the brain (striate V1 region of the visual cortex) at days three and 15 post-injection compared to baseline (See Figure 1).
- The initial dose was safe and well-tolerated at day 29, with no adverse events reported throughout the study.
- The duration of effect is consistent with preclinical pharmacokinetic data.
Eric Daniels, MD, MBA, chief development officer of Kiora Pharmaceuticals, pointed out the early results demonstrate profound improvements in light perception and functional vision.
A simulation of vision of a person living with retinitis pigmentosa.

“As a confirmatory measure, and being an open label study, we reviewed preliminary observations from the first patients treated and felt it was important to share these early findings,’ he added in the news release. “The collective body of data supports our plan to escalate doses, complete enrollment, and report full results to the medical and patient communities later this year."
According to the news release, retinitis pigmentosa is a group of rare, inherited diseases that involve a breakdown and loss of the photoreceptor cells in the retina, the light-sensing tissue that lines the back of the eye. This progressive disease usually initially presents with difficulty seeing at night and a loss of side (peripheral) vision, extending to tunnel vision and eventually, full blindness.
Brian M. Strem, PhD, president and CEO of Kiora Pharmaceuticals, pointed out there currently are no approved therapies to help patients with retinitis pigmentosa, which is estimated to affect 100,000 people in the U.S. alone.
“We see KIO-301 as a potential treatment option across all genetic causes of retinitis pigmentosa as an easy-to-deliver small molecule,” he said in the release. “Based on how KIO-301 works as a light sensing photoswitch, we also see it potentially addressing additional eye diseases, including other inherited and age-related retinal degenerative diseases, namely geographic atrophy and late-stage wet AMD."
The trial, called the ABACUS study, is a Phase 1b open-label, single ascending dose clinical trial for people living with retinitis pigmentosa. The study comprises the enrollment of six patients and the evaluation of 12 eyes, following monitoring for 29 days. The first cohort of three patients includes individuals with no or bare light perception due to the progression of RP. The second cohort includes three patients able to detect hand motion and count fingers. The primary endpoints are safety and tolerability, with secondary efficacy endpoints including object identification and contrast assessment, navigation, fMRI and other ophthalmic and quality-of-life assessments. This study is being conducted at multiple sites in Adelaide, South Australia, including The Royal Adelaide Hospital.
KIO-301 is a visible light-sensitive small molecule that acts as a reversible 'photoswitch', specifically designed to restore the eyes' ability to perceive and interpret light in visually impaired patients. KIO-301 selectively enters retinal ganglion cells (those downstream of degenerated rods and cones) and 'switches' them into light sensing cells, capable of signaling the brain as to the presence or absence of visible light.
Newsletter
Don’t miss out—get Ophthalmology Times updates on the latest clinical advancements and expert interviews, straight to your inbox.





