Article
Connecting the dots between the complement pathway and geographic atrophy
Author(s):
Currently enrolling a Phase 2 trial to evaluate the role of complement factor B protein in age-related macular degeneration (AMD)
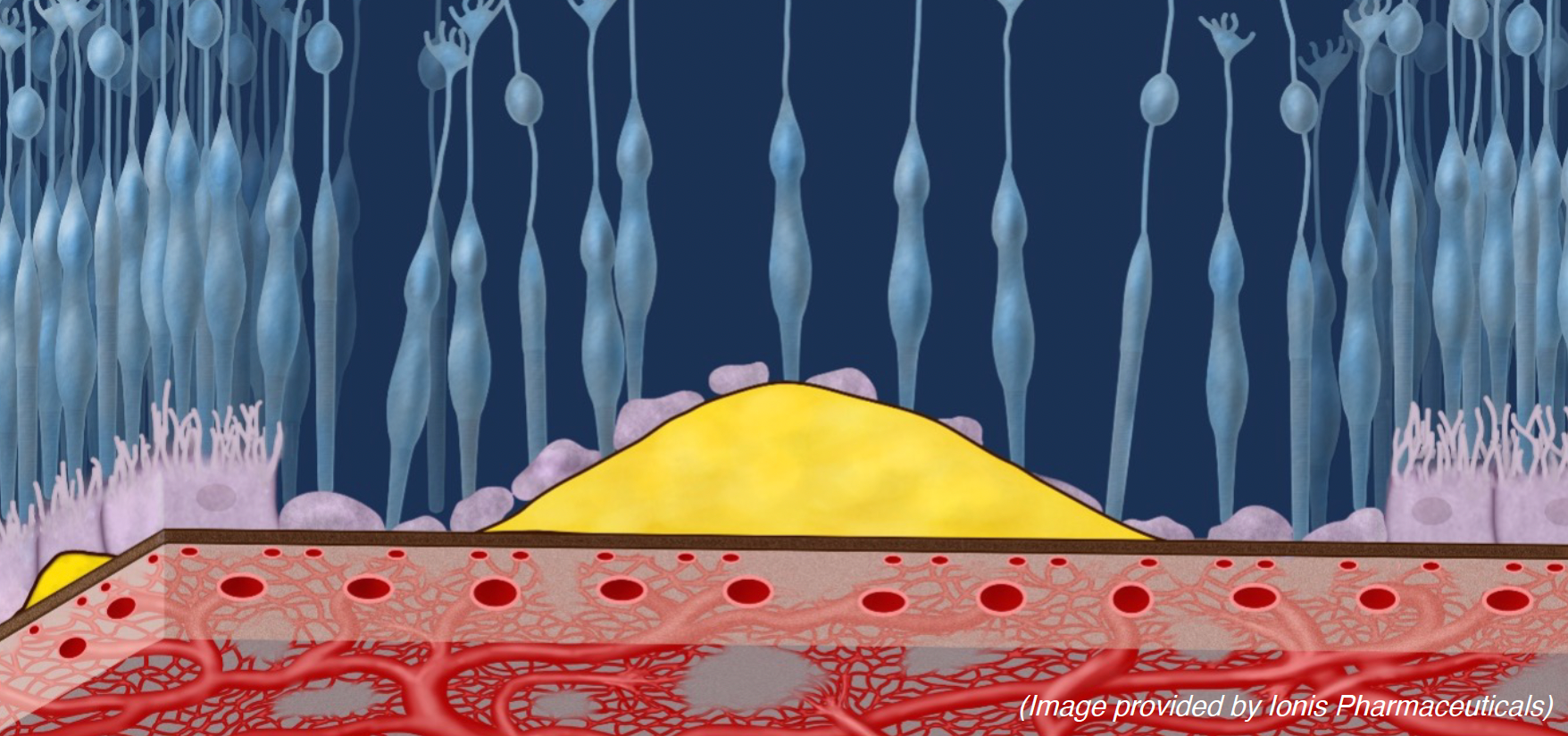
Image displaying extracellular deposits (drusen) and thinning of the choriocapillaris.
The GOLDEN Study
Ionis Pharmaceuticals is currently enrolling patients in the GOLDEN Study, a Phase 2 clinical trial investigating the safety and effectiveness of IONIS-FB-LRx for GA secondary to AMD.
Patients in the United States aged 50 years or older with a medical diagnosis of AMD and GA in at least one eye may prequalify to participate in the GOLDEN Study.
The study involves a screening period to determine whether patients can safely participate based on their medical history and current health. For those enrolled, there will be a 45-week treatment period and a 12-week follow-up period. Numerous precautions are in place to ensure the health and well-being of all current and potential clinical trial participants, given the COVID-19 pandemic.
It has been very frustrating to watch vision loss due to age-related macular degeneration in my patients without a good treatment available to help them. The path that Ionis has taken allows both eyes to be treated at the same time, sparing patients the need for intravitreal injections. The science behind this is very strong and targets the pathway that appears to be associated with progressive vision loss. I am hopeful that this therapy will lead to improved vision quality for patients living with geographic atrophy. — David S. Boyer, M.D., Senior Partner, Retina-Vitreous Associates Medical Group
An investigational RNA-targeted approach to address one of the root causes of dry AMD
We have seen RNA-based vaccines be hailed as scientific saviors over the last two years in response to COVID-19. However, the potential of RNA-targeted therapies to treat and prevent a variety of disease has long been recognized by scientists.
The RNA-targeted investigational therapeutic, IONIS-FB-LRx, leverages antisense medicine to modulate the overactivity of the complement system. Specifically, IONIS-FB-LRx seeks out, binds to and destroys the specific messenger RNA (mRNA) in the liver to reduce the production of complement factor B protein that is found in the blood and eyes, and directly addresses one of the root causes of GA.
IONIS-FB-LRx is delivered by a single monthly subcutaneous injection in the abdomen or thigh, rather than in the eye, and has the potential to treat both eyes with a single dose. This is particularly important given people with GA in one eye are more likely to develop it in the other.1
Pre-clinical studies have already shown promising results in cynomolgus monkeys treated for 13 weeks. Systemic antisense oligonucleotide (ASO) administration resulted in a reduction of ocular and systemic complement factor B protein.2
Background information: AMD, GA and the complement system
In the U.S., an estimated 2.07 million people are affected by AMD.3 By 2050, this number is expected to more than double to 5.44 million.3
The development of a variety of complement-mediated diseases throughout the body, including dry AMD, is strongly associated with part of the body’s immune system called the “complement system.” Over-activation of complement pathways allows the complement system to damage host cells – in this case, the retina or more specifically the retinal pigment epithelium (RPE). This damage is cumulative so that the greatest predictive risk factor in vulnerable individuals is age.3 Additionally, studies have observed systemic complement factor B protein circulating in choriocapillaris and the posterior retina as retinal degeneration occurs.4
GA in AMD is characterized by the accumulation of extracellular deposits (drusen) and thinning of the choriocapillaris, leading to the formation of islands of photoreceptor and RPE loss that rapidly enlarge and encircle the fovea until the central vision is lost.4,5 GA has been shown to have modulating local complement pathway activity at sites of degeneration of the retina and choriocapillaris.
There are currently no proven treatment options to arrest or reverse GA. However, a greater understanding of the role of complement factor anomalies in the causation of AMD gives hope that complement targeted therapy could address the underlying cause and give patients with advanced cases of dry AMD a better chance to preserve one of the most important senses for navigating daily life.
Talk to your GA patients about their potential to participate in the GOLDEN Study
If you know a patient who fits the above criteria and you would like to refer them for enrollment in the GOLDEN Study, please contact us at ionisNCT03815825study@clinicaltrialmedia.com or call 844-414-1686.
To learn more about the clinical trial and eligibility, visit ionistrials.com/golden-study. IONIS-FB-LRx is an investigational therapy and has not been approved for use by any regulatory authority.
About Ionis Pharmaceuticals
For more than 30 years, Ionis has been the leader in RNA-targeted therapy, pioneering new markets and changing standards of care with its novel antisense technology.
---
