
Nanoscope Technologies LLC announced this week it has received Direct Phase II SBIR grant from National Institutes of Health for developing an innovative approach to autonomously regulate pressure for treatment of Glaucoma in a gene-agnostic manner.

Nanoscope Technologies LLC announced this week it has received Direct Phase II SBIR grant from National Institutes of Health for developing an innovative approach to autonomously regulate pressure for treatment of Glaucoma in a gene-agnostic manner.
In an announcement at the American Academy of Ophthalmology’s 2022 annual meeting at McCormick Place in Chicago, the company noted that results from Phase 1/2 MGT009 study demonstrate safety profile of investigational gene therapy botaretigene sparoparvovec (AAV-RPGR) and suggest sustained vision improvement in patients with X-linked retinitis pigmentosa.

Recent reports of retinal atrophy have raised concerns on potential long-term safety.

LUNA trial will evaluate the 2x10^11 vg/eye (2E11) and a new lower 6x10^10 vg/eye (6E10) dose of Ixo-vec, with enhanced prophylactic steroid regimens in patients requiring frequent anti-VEGF injections. Interim data anticipated throughout 2023.

Nanoscope Therapeutics’ Phase 2 STARLIGHT open-label trial enrolled 6 subjects with advanced vision loss due to a clinical or genetic diagnosis of Stargardt disease.
Scientist have proposed several methods for converting stem cells into RPE, but there is still a gap in our knowledge of how cells respond to these stimuli over time.

If approved, TP-03 may offer treatment for millions of patients with Demodex blepharitis. TP-03 is now also being studied for the treatment of Meibomian Gland Disease in patients with Demodex mites.
According to a university news release, its CellSight teams clinched top two of three awards in National Eye Institute competition.

Researchers in Trinity’s School of Genetics and Microbiology developed a new gene therapy, ophNdi1, that is the first of its kind to directly target mitochondrial function in cells that are malfunctioning in AMD.

Gene therapy has partly restored the function of the retina’s cone receptors in two children who were born completely colorblind, reports a new study led by UCL researchers.

Stoke Therapeutics announced enrollment of the first patient in a prospective natural history study of people ages 8 to 60 who are living with autosomal dominant optic atrophy.
Low-grade inflammation seems to be associated with baseline disease in inherited retinal dystrophies (IRDs), according to Christine N. Kay, MD, a surgeon at Vitreoretinal Associates, in Gainesville, Florida.

APOE4 gene associated with Alzheimer’s disease risk was found to protect mice from glaucoma. Research team also prevented retinal ganglion cell death by blocking the APOE signaling pathway, pointing to a potential treatment strategy for glaucoma.

X-linked retinoschisis is a congenital disorder that results in visual impairment in boys at an early age. No treatment is currently available, and surgical repair fails in many cases.

The study provided evidence of safety, visual acuity improvement and structural stability in a difficult-to-treat patient population.

According to a news release, the U.S. Food and Drug Administration has approved Coherus’ ranibizumab-eqrn (Cimerli) as an interchangeable biosimilar for all five indications of Lucentis.

A multi-institutional effort led by researchers at the University of Pennsylvania is taking steps to develop an effective technique to regenerate photoreceptors cells and restore sight in patients with vision disorders.

The discovery of molecular signatures of age-related macular degeneration will help with better diagnosis and treatment of this progressive eye disease.
Scientists at the Louisiana State University Health New Orleans Neuroscience Center of Excellence have developed a new, experimental human cell line from retinal pigment epithelial cells.

According to the company, 6-month safety and efficacy data are expected in Q1 2023. MCO-010 gene therapy reprograms healthy retinal cells to make them photosensitive.
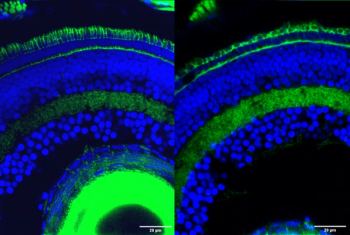
The findings could lead to a new understanding of unexplained causes of heritable retinal diseases.

Pediatric ophthalmologists may need background information when treating young patients.

Eyes of mice lacking protective protein show signs similar to age-related macular degeneration.

According to the company, the primary outcome of the MGT009 study is safety, and botaretigene sparoparvovec treatment was found to be generally safe and well-tolerated.

Jason Menzo is named CEO, while Russell Kelley, PhD, MBA, will become managing director of the RD Fund. Ben Yerxa, PhD, will take the helm of Opus Genetics, the first RD Fund- and foundation-backed spin-off company.