Dry Eye
Latest News

Researchers receive $6.7 million in federal grants to develop technology to monitor, treat chronic eye diseases
Latest Videos

CME Content
More News

Delivered in a water-free solution, Vevye’s new formulation offers a high concentration of cyclosporine that works rapidly, has no preservatives, and rarely causes irritation

TL-925 is a first in class, topical Bruton’s tyrosine kinase (BTK) inhibitor that prevents mast cell activation and degranulation, and also blocks the cytokine-driven inflammatory response, and restores lacrimal unit homeostasis, offering the potential for disease modification

In the trial, PL9643 failed to meet co-primary and secondary endpoints.

Products under the terms of the agreement include VERKAZIA, Cationorm PLUS, VEVYE, IHEEZO, and ZERVIATE.

The recall is “due to potential safety concerns after FDA investigators found insanitary conditions.”

AR-15512, a topical transient receptor potential melastatin 8 agonist, is a first-in-class product candidate for the treatment of the signs and symptoms of dry eye disease. Alcon plans to file a New Drug Application for AR-15512 with the FDA in mid-2024.

According to the company, cyclosporine ophthalmic solution 0.1% is the first and only cyclosporine-based product for treating the signs and symptoms of dry eye disease.

According to OKYO Pharma, the first-in-human, randomized, double-masked, placebo-controlled trial of OK-101 “established a clear and informed path for further development in Phase 3 registration trials.”
The company is announcing 3 market access partnerships with PhilRx, Apollo Care, and PARx Solutions, expanding US availability of the solution.

DED incidence rates vary markedly depending on the ophthalmic practice.

The company announced plans to enroll 320 volunteers in the study. Vezocolmitide is the first drug candidate based on PolyCol, Stuart Therapeutics' patented synthesized polypeptide collagen mimetic peptide platform.
Interventional eyelid procedures enabled by TearCare technology delivered clinically and statistically significant improvements in every sign and symptom measured at every time point through 6 months.

The companies announced they will team up to enhance patient care and workflow efficiencies with a comprehensive dry eye assessment and relief platform.

According to the company, its RELIEF Phase 2b trial will evaluate the efficacy and safety of licaminlimab in moderate-to-severe dry eye disease, and further explore the potential of a genetic biomarker. Topline results are expected in mid-2024.

The company says top-line efficacy and safety data is on track for release in December of 2023.

According to the company, an additional trial is required to show positive effects on the treatment of ocular symptoms in dry eye disease.
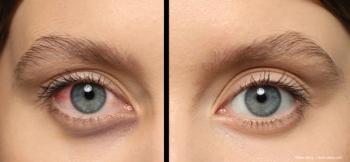
Slit lamp evaluation can provide valuable information for treatment course.

This is just one of many times the FDA has issued a warning to companies for the sale of unapproved ophthalmic products in 2023.

By taking a proactive approach to treatment, ophthalmologists can identify the “silent sufferers.”

Ophthalmologists end their discussion on the management of dry eye disease with other ocular comorbidities highlighting key takeaways and prospective advances in care and outcomes.

Experts offer clinical advice and when to refer patients to specialists and the importance of building a supportive care network for patients.
Ophthalmologists offer their clinical insights and best practices of procedural therapies for dry eye management prior to surgery.

Experts discuss how dry eye disease can impact the preparation and post-operative care of cataract or refractory surgery, highlighting key approaches to successfully managing these patients.

Experts discuss their experience managing patients with dry eye disease and glaucoma, highlighting how dry eye disease impacts the progression of glaucoma and key considerations for treatment.

Ophthalmologists provide insights into their diagnostic protocols for assessing dry eye.


































