- COVID-19
- Biosimilars
- Cataract Therapeutics
- DME
- Gene Therapy
- Workplace
- Ptosis
- Optic Relief
- Imaging
- Geographic Atrophy
- AMD
- Presbyopia
- Ocular Surface Disease
- Practice Management
- Pediatrics
- Surgery
- Therapeutics
- Optometry
- Retina
- Cataract
- Pharmacy
- IOL
- Dry Eye
- Understanding Antibiotic Resistance
- Refractive
- Cornea
- Glaucoma
- OCT
- Ocular Allergy
- Clinical Diagnosis
- Technology
Real-world outcomes validate intraocular drug delivery method
Ophthalmologists seek methods to control postoperative inflammation.
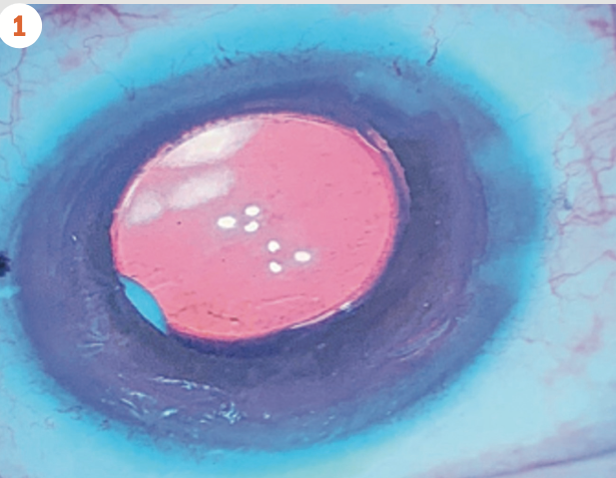
(Figure 1) Dexamethasone intraocular suspension 9% in the posterior chamber at the end of cataract surgery.(Image courtesy of Michael Savetsky, MD)
Special to Ophthalmology Times®
Mitigating inflammation after cataract surgery has always been critical, but heightened expectations among today’s refractive cataract surgery patients reinforce that imperative.
Despite steady advances in surgical techniques, postoperative inflammation, if uncontrolled, may result in discomfort, delayed recovery, and reduced visual outcomes.1,2
Although topical corticosteroids and nonsteroidal anti-inflammatories (NSAIDs) have been the historical standard for inflammation control following ocular surgery, the likelihood of inconsistent patient adherence to these medications is high, and this behavior is associated with compromised outcomes.
The need for targeted anti-inflammatory treatments that minimize the challenges associated with topical dosing and patient adherence has given rise to innovative drug-delivery systems, such as dexamethasone intraocular suspension 9% (Dexycu; EyePoint Pharmaceuticals, Inc), the first single-dose, sustained-release intraocular corticosteroid FDA approved for inflammation following ocular surgery.
This liquid suspension is injected at the end of ocular surgery using a standard 27-gauge cannula; after an immediate bolus, the approximately 2-mm spherule delivers a tapering dose of dexamethasone to control inflammation through the first postoperative month.
Clinical study outcomes
Impressive phase 3 clinical study outcomes influenced my initial decision to offer this drug-delivery option to my patients.3,4
In one phase 3, randomly assigned, double-masked, placebo-controlled trial, significantly more dexamethasone intraocular suspension 9%-treated eyes (66%) had anterior chamber cell clearing at postoperative day 8 vs 25% of those given placebo (vehicle injection).3
In another phase 3, open-label safety study, dexamethasone intraocular suspension 9% was found comparable to 4-times-daily topical prednisolone drops.4
The most common adverse events were increased IOP (11.1%), iritis (6.3%), and systemic effects (7.9%, dexamethasone intraocular suspension 9% group; 10.9%, prednisolone group). Among the patients receiving dexamethasone intraocular suspension 9%, 68.7% strongly agreed that not having to use eye drops was very convenient.4
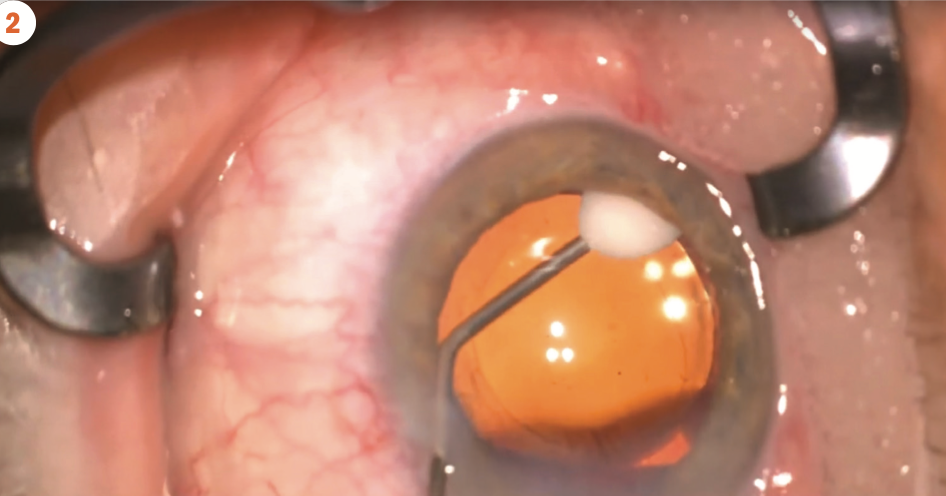
(Figure 2) Dexamethasone intraocular suspension 9% in the capsular bag at the end of cataract surgery.(Image courtesy of Robert Osher, MD)
Real-world outcomes
Further evidence validating my use of this intraocular delivery method has emerged from a multicenter retrospective study of the real-world performance of dexamethasone intraocular suspension 9%.5
Early experiences
The investigators aimed to characterize surgeons’ early experiences with dexamethasone intraocular suspension 9% using data from a large number of patients and study sites.
Data were collected from records of 641 eyes of 527 patients who received the agent at the end of cataract surgery between March and December 2019, the months immediately following the drug’s market availability.
In this retrospective study, among those eyes with a recorded value at each time point, complete anterior chamber cell clearing was observed in 40%, 65%, 85%, and 90% at postoperative days 1, 8, 14, and 30, respectively.
Targeted best corrected visual acuity was achieved in 97% of eyes. Mean IOP at postoperative day 1 was 18.6 mm Hg, with levels decreasing through to postoperative day 30.
These real-world data from multiple US study centers demonstrate the anti-inflammatory efficacy and tolerability of dexamethasone intraocular suspension 9% and mirror those of the controlled phase 3 clinical trials.4,5
Viewing these real-world results alongside those from the phase 3 registration trials can be illustrative, especially regarding the patient population included.
FDA studies generally follow a fairly restrictive set of inclusion and exclusion criteria, whereas this real-world retrospective study included all patients at participating centers who had received dexamethasone intraocular suspension 9% during the allotted time period, giving a window into the drug’s performance in an “unfiltered” population, including patients with complicated ocular histories and comorbidities.
Although there are some limitations to this type of real-world retrospective analysis, the insight gained can be very powerful.
Patient selection
Data from the aforementioned studies, as well as my own anecdotal observations, form the basis of my dexamethazone intraocular suspenson 9% patient selection standards today.
In my practice, 95% of cataract and combined cataract and minimally invasive glaucoma surgery (MIGS) procedures conclude with dexamethasone intraocular suspension 9% administration.
Although the pivotal trials for the drug were conducted in cataract surgery, it is approved for any ocular surgery and is now routinely used for postoperative inflammation control in MIGS and even vitreoretinal surgeries.6
Some may wonder whether the option is good for every patient. It certainly has its merits, and been proven safe.
I consider nearly every patient to be a candidate for dexamethasone intraocular suspension 9%, based on its efficacy and safety profile.
I find its ability to quell postoperative inflammation comparable to difluprednate ophthalmic emulsion 0.05% topical corticosteroid drops (Durezol; Novartis), which I previously relied on for the majority of my patients with cataract and cataract/MIGS.
Patients who may not be appropriate candidates for dexamethasone intraocular suspension 9% include those who are likely to need their steroid dosage modified at a moment’s notice (eg, known steroid responders and patients who have advanced glaucoma).
For known steroid responders and those with advanced glaucoma, I would prefer to use a topical anti-inflammatory regimen I could alter as needed.
IOP elevations
However, in my experience with dexamethasone intraocular suspension 9%, IOP elevations in patients without additional risk factors may occur within the first few postoperative days and resolve without intervention by the end of the first week.
In general, risk of severe or prolonged IOP elevation in patients treated with the agent is low; a post hoc, pooled analysis of phase 3 study data found the risk of postoperative IOP elevation with dexamethasone intraocular suspension 9% was comparable to topical steroid therapy.7
Complicated patients
Dexamethasone intraocular suspension 9% has particular utility in patients with complex histories and/or inflammatory risk factors.
Prior to the availability of this sustained-release formulation, I would occasionally administer a small amount of dexamethasone intracamerally to provide a boost of anti-inflammatory prevention in patients at risk of elevated postoperative inflammation, such as those with uveitis.
Now I notice that when I administer dexamethasone intraocular suspension 9% to a patient with a history of uveitis, I see excellent inflammation control without the use of adjunctive topical steroid.
Intraoperative iris bleed
As another example, I recently operated on a patient with diabetes who had an intraoperative iris bleed. I was able to wash out about 95% of the blood cells from the anterior chamber but could not clear the remainder.
I injected dexamethasone intraocular suspension 9% into the posterior chamber and saw the patient at 1, 3, and 7 days postoperatively. By day 3, he had almost no cells in the anterior chamber, and by 1 week he was fully clear.
This is the type of patient whom I would normally expect to experience significant inflammation and possibly even require additional postoperative steroid eye drops, but neither of those occurred, which speaks to the potency of dexamethasone intraocular suspension 9%.
In addition to using dexamethasone intraocular suspension 9%, I continue to prescribe a once-daily topical NSAID, either bromfenac ophthalmic solution 0.07% (Prolensa; Bausch + Lomb) or nepafenac ophthalmic suspension 0.3% (Ilevro; Novartis).
One drop is easy for the patient to remember, and I have always given an NSAID postoperatively for prophylaxis of cystoid macular edema.
Conclusions
The use of dexamethasone intraocular suspension 9% takes the responsibility of postoperative inflammation out of the patients’ hands, which is helpful to them and me.
They gain the convenience that comes with not having to procure topical steroids, administer them correctly, or remember to use them multiple times a day, according to the tapering schedule.
I benefit because I can be sure that patients have anti-inflammatory coverage in place, which in turn improves surgical outcomes.
--
About the author
Michael Savetsky, MD
p: 323/728-5500
e:SavetskyMD@gmail.com
Savetsky is a board-certified cornea/external disease, cataract, and refractive surgeon specializing in laser refractive surgery, corneal transplants, and advanced cataract surgery. He is in private practice in Los Angeles, California. Savetsky is a consultant for EyePoint Pharmaceuticals.
---
References
1. McColgin AZ, Heier JS. Control of intraocular inflammation associated with cataract surgery. Curr Opin Ophthalmol. 2000;11(1):3-6. doi:10.1016/j.ophtha.2017.12.029
2. Mentes J, Erakgun T, Afrashi F, Kerci G. Incidence of cystoid macular edema after uncomplicated phacoemulsification. Int J Ophthalmol. 2003;217(6):408-412. doi:10.1159/000073070
3. Donnenfeld E, Holland E. Dexamethasone intracameral drug-delivery suspension for inflammation associated with cataract surgery: a randomized, placebo-controlled, phase III trial. Ophthalmology. 2018;125(6):799-806. doi:10.1016/j.ophtha.2017.12.029
4. Donnenfeld ED, Solomon KD, Matossian C. Safety of IBI-10090 for inflammation associated with cataract surgery: phase 3 multicenter study. J Catract Refract Surg. 2018;44(10):1236-46. doi:10.1016/j.jcrs.2018.07.015
5. Weinstock R. Dexamethasone intraocular suspension 9% after cataract surgery: data from a retrospective study. Presented at: American Academy of Ophthalmology Annual Meeting; November 13-15, 2020; virtual.
6. Kiernan DF. Dexamethasone intracameral drug-delivery suspension for inflammation associated with vitreoretinal surgery. BMJ Open Ophthalmology. 2020;(5)e000491. doi:10.1136/bmjophth-2020-000491
7. Matossian C, Hovanesian J, Bacharach J, Paggiarino D, Patel K. Impact of dexamethasone intraocular suspension 9% on intraocular pressure after routine cataract surgery: post hoc analysis. J Cataract Refract Surg. 2020;47(1):53. doi:10.1097/j.jcrs.0000000000000363

